Francisco Cesar Carnevale • Airton Mota Moreira
BACKGROUND
Benign Prostatic Hyperplasia
Symptomatic benign prostatic hyperplasia (BPH) typically occurs at the beginning of the sixth decade, with more than 40% of men aged 60 years and older presenting with clinical manifestations. As the world’s population ages, the prevalence of BPH is expected to increase, calling for a therapy that minimizes the risk for adverse outcomes.1–3
The standard management of BPH is based on the overall health of the patient, on the severity of the lower urinary tract symptoms (LUTS), and on quality-of-life considerations. Voiding difficulties attributable to BPH can be quantified with the American Urological Association Symptom Index (AUA-SI) score or International Prostate Symptom Score (IPSS). Various medications can decrease the severity of voiding symptoms secondary to BPH. Impotence, decreased libido, and ejaculatory disorders are known side effects of these medications. Watchful waiting is a management strategy in which the patient is monitored by his physician but receives no active intervention for BPH; this is the preferred management strategy for patients with mild symptoms.4
The American Urological Association (AUA) guidelines indicate that patients with mild LUTS secondary to BPH (AUA-SI score <8) and patients with moderate or severe symptoms (AUA-SI score ≥8) who are not bothered by their LUTS should be managed using a strategy of watchful waiting. If the patient elects interventional therapy and there is sufficient evidence of obstruction, the patient and urologist should discuss the benefits and risks of the various available interventions.4
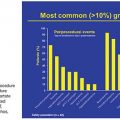
Many advances have been made in the medications and minimally invasive procedures used to treat BPH since the 1990s. Despite these advances, transurethral resection of the prostate (TURP), which was developed in the 1920s, remains the treatment of choice when medical management fails. TURP is still the gold standard of interventional treatment for small prostate glands (smaller than 80 to 100 g) and is performed under direct endoscopic visualization with an electrocautery tool to remove prostatic tissue. Although considered a safe technique with a mortality rate below 0.25%, it is not without adverse events. The most frequent complications are ejaculatory disorders (up to 50%), early urinary incontinence (30% to 40%), acute urinary retention caused by blood clots (2% to 5%), sexual impotence (up to 5%), and the need for blood transfusions (0.4% to 7%). Patients who have undergone TURP require surgical retreatment for LUTS symptoms in 3% to 14.5% of cases.4–6
Open prostatectomy involves the surgical removal (enucleation) of the inner portion of the prostate gland via a suprapubic or retropubic incision in the lower abdomen. Open prostatectomy is typically performed on patients with prostate volumes greater than 80 to 100 mL with a higher risk of blood loss and transfusion and a longer hospital stay than TURP. Open prostatectomies may only be needed for men with very enlarged prostate glands (it may be more effective than TURP in relieving the blockage of urine flow) and for men with bladder diverticula (pockets) or stones.7,8
The high prevalence rate of BPH has a tremendous impact on the health and quality of life of men. Increasingly, BPH therapy trends are moving away from the standard operations toward less invasive pharmacologic options and minimally invasive procedures provided in an outpatient setting. Minimally invasive techniques have been developed as alternative treatments for LUTS, such as transurethral microwave thermotherapy and laser ablations, but they involve introducing energy into the gland and all require access through the urethra. Nevertheless, complications from these procedures are similar to TURP.9–11 Prostatic artery embolization (PAE) has emerged as a new minimally invasive alternative of treatment for symptomatic patients with enlarged prostates due to BPH.12–14
Prostatic Artery Embolization
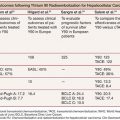
The first animal study to evaluate the feasibility and safety of PAE was conducted in a canine model and demonstrated the promising potential of PAE to decrease prostate volume and urethral stenosis due to BPH. The success rate for identification and selective catheterization of the prostatic arteries was 100%. Initial computed tomography (CT) showed good distribution of particles in the embolized prostate with no evidence of nontarget embolization. CT after 1 month showed decreased perfusion, cavitary necrosis, and 40% prostate volume reduction. There was excellent radiologic–pathologic correlation.15 Subsequently, the technical feasibility and safety of prostate embolization was evaluated in pigs16 and dogs17 with prostate volume reduction and without sexual or erectile complications.
Embolization of the prostatic arteries has been used for many years to control serious bleeding after biopsy or prostatectomy.18–20 The first published case in which it was recognized that PAE could have a therapeutic effect on BPH was in 2000 by DeMeritt and colleagues.12 The first intentional treatment of BPH with PAE in humans was done by Carnevale et al.13 in June 2008 and was published in 2010. In this report, PAE was performed in two patients with acute urinary retention due to BPH who were refractory to treatment with selective α-blockers, were being managed with long-term indwelling catheters, and were waiting for surgery. The same investigators published midterm follow-up data for these two patients in 2011, confirming the efficacy of the procedure.14
TECHNICAL DETAILS
Preprocedure Imaging Evaluation
Ultrasound (US) is the most common method to evaluate the prostate and bladder. The prostate volume is measured by standard transabdominal view or transrectal US if prostate biopsy is necessary. Prostate vascularization can be visualized using Doppler, and transabdominal US can measure the postvoid residual urine volume. Bladder evaluation comprises measurement of wall thickness and an assessment of characteristics such as the presence of diverticula, trabeculations, and other possible findings such as polyps, calculus, or other lesions. Protrusion of the median lobe is measured using the prostate protrusion index and prostate volume is measured using three incidences.
Magnetic resonance imaging (MRI) of the prostate is very useful, giving more details than US mainly related to the central gland and peripheral zone. Prostate measurements (cephalocaudal, transverse, and anteroposterior) are obtained and volume is calculated by the ellipse formula. It is the best method to evaluate patients before and after PAE. CT with contrast can be useful to identify the characteristics of the main artery supplying the prostate and any arterial atherosclerotic lesion or obstruction that could contraindicate or make the intervention more difficult.21
Embolization Technique
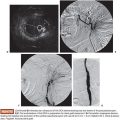
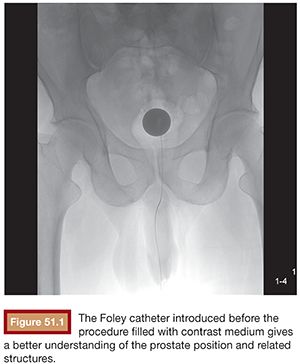
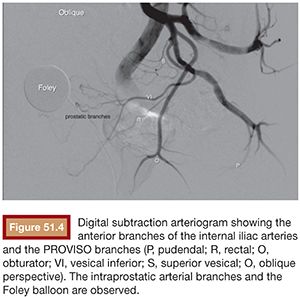
BPH treatment with PAE requires a well-trained interventional radiologist because of the complex prostatic vascular anatomy and the potential for complications in elderly patients with atherosclerosis, very small prostatic arteries, and comorbidities. Intervention can be performed under local anesthesia on an outpatient basis. A 400 mg intravenous dose of ciprofloxacin is given before the procedure followed by 500 mg orally twice a day for 7 days after PAE. Patients also receive nonopioid analgesics and nonsteroidal anti-inflammatory medications after embolization. Intervention can be performed via a unilateral common femoral arterial approach. Femoral pulses should be examined before choosing which side will be used for access. To provide good orientation to the prostate site and related structures in the pelvis, we recommend that a Foley catheter be placed into the bladder and that the balloon be filled with a mixture of 30% iodinated contrast medium and 70% normal saline (Fig. 51.1). This is used during the procedure to give both a better image and understanding of the prostate, the internal iliac artery branches, and related structures (Fig. 51.2) to help avoid nontarget embolization complications. It is an excellent landmark during the procedure and we have not observed any severe complications due to its use. It can also be a reference if using cone-beam CT and can help eliminate the patient’s need to strain to void after the procedure.
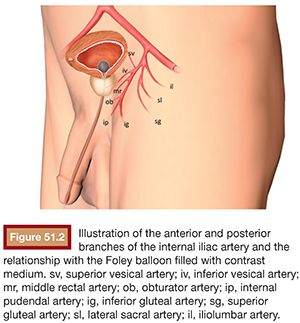
Knowing the vascular anatomy of the prostate is essential for the successful performance of this procedure. Due to many arterial anastomoses and branches to related pelvic structures, various nomenclatures have been used for the arteries supplying the prostate. Names such as the inferior vesical artery (IVA), prostatovesical artery, vesicoprostatic artery, and prostatic artery have been used.21,22 According to the urologists’ nomenclature, the IVA has traditionally been considered the main prostatic artery. It usually arises as the second or third branch of the anterior trunk of the internal iliac artery. Generally, one main prostatic artery is found on each side in that position, but the main prostatic artery or additional prostatic branches arising from the superior vesical, internal pudendal, obturator, and middle rectal arteries can also be found in some patients.23
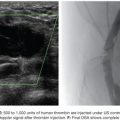
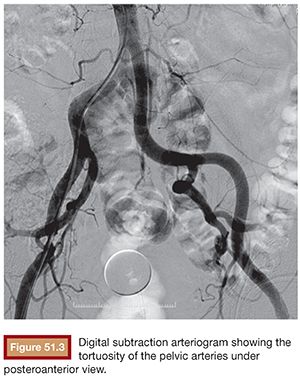
Initial pelvic angiography is performed (20 mL; 10 mL per second) to evaluate the iliac vessels and the prostate arteries during arterial and late phases (Fig. 51.3). After crossing the aortic bifurcation, selective digital subtraction arteriogram of the internal iliac artery is performed with a 5-Fr vertebral or Cobra 2 (Merit Medical System, Inc., South Jordan, UT) catheter (12 mL; 4 mL per second) to better assess the blood supply to the prostate. The 5-Fr diagnostic catheter should be placed at the common internal iliac trunk to avoid missing any branch arising from the anterior and posterior division. It is not uncommon to find an IVA arising as a common pedicle with the superior vesical artery (also called umbilical artery) as the first branch of the anterior division. The 5-Fr Roberts Uterine Catheter (RUC; Cook Medical, Inc., Bloomington, Indiana) can be used but, due to its long curve, we have observed less torquability and pushability with the microwire when using the coaxial system. For the ipsilateral internal iliac artery catheterization, a Simmons 1 or 2 catheter (Merit Medical System, Inc., South Jordan, UT) can be used, but we prefer to work with the same vertebral catheter making a Waltman loop because, if necessary, it can be used as a regular vertebral catheter to get more torquability and pushability.
The best projection in which to identify the IVA and all possible accessory branches to the prostate is the 25- to 55-degree ipsilateral oblique view. A caudal view (10 to 20 degrees) can help to identify some bladder branches. This perspective, with the help of the Foley balloon filled with dilute contrast, gives a better understanding of all five anterior branches and gives orientation to the prostatic arteries. After performing the internal iliac arteriogram, attention should be given to the arteries feeding the area immediately below the balloon. In this view, the branches of the anterior division will be straightened and the prostate branches and the rectal branches stay in the anterior and posterior position, respectively. The PROVISO acronym (internal Pudendal, middle Rectal, Obturator, Vesical Inferior, and Superior under Oblique view) is a very useful trick to remember the names of the arteries during arteriography (Fig. 51.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree