Diagram 1
Distribution and proportions of the tissue layers composing the prostate. Diagram of the prostate shows its zonal anatomy in the sagittal plane and corresponding axial sections from the base (upper line), midgland (medium line), and apex (lower line). Note the anterior fibromuscular stroma (dark gray part in the anterior portion), peripheral zone (light gray), central zone (white), and transition zone (dark gray)
7.1.2 Introduction and Pathogenesis
Benign prostatic hyperplasia (BPH) represents the most commonly diagnosed urological disease in men after the fifth decade. Although BPH etiology remains uncertain in some aspects, several mechanisms have been proposed to be involved in the pathogenesis and progression of BPH.
First, aging represents the most significant risk factor for the development of BPH and the compliant of lower urinary tract symptoms (LUTS). In aging men, an interference in growth factor pathway occurs and a significant tissue-remodeling process takes place, leading to prostatic enlargement. Second, hormonal alterations have been proposed to be involved in BPH pathogenesis and progression. Indeed, the development of BPH requires the presence of testicular androgens, and BPH tissue has higher dihydrotestosterone activity than normal prostate gland tissue.
Additionally, insulin resistance with secondary hyperinsulinemia has been proposed to be involved in the development of BPH. Third, a role of increased sympathetic nerve activity has also been proposed. Finally, in the last few years, the role of prostatic inflammation as a crucial part of BPH pathogenesis and progression has emerged. The chronic inflammatory condition may contribute to tissue injury, activating cytokine release and increasing the concentration of growth factors, creating a local vicious cycle. In this context, the upregulation of proinflammatory cytokines has been widely reported in prostatic tissues of patients with BPH. Concluding, all these data support the hypothesis that tissue damage, hypoxia, and chronic process of wound healing lead to a persistent process of stimulation of stromal and epithelial prostatic tissues, potentially resulting in BPH [2, 3].
Risk factors for the development of BPH are poorly understood. Some studies have suggested a genetic predisposition, and some have noted racial differences. Approximately 50 % of men under the age of 60 who undergo surgery for BPH may have a heritable form of the disease. This form is most likely an autosomal dominant trait, and first-degree male relatives of such patients carry an increased relative risk of approximately fourfold.
Interestingly, it has been hypothesized that inflammatory infiltrate leads to tissue damage and to a chronic process of wound healing that might subsequently determinate prostatic enlargement [4].
7.1.3 Epidemiology
BPH is the most common benign tumor in men, and its incidence is age related. The prevalence of histological BPH in autopsy studies rises from approximately 20 % in men aged 41–50 to 50 % in men aged 51–60 and to >90 % in men older than 80. Although clinical evidence of disease occurs less commonly, symptoms of prostatic obstruction are also age related. At age 55, approximately 25 % of men report obstructive voiding symptoms. At age 75, 50 % of men complain of a decrease in the force and caliber of their urinary stream [5, 6].
7.1.4 Clinical and Laboratory Findings
The symptoms of BPH can be divided into obstructive and irritative complaints. Obstructive symptoms include hesitancy, decreased force and caliber of stream, sensation of incomplete bladder emptying, double voiding (urinating a second time within 2 h of the previous void), straining to urinate, and post-void dribbling. Irritative symptoms include urgency, frequency, and nocturia. A detailed history focusing on the urinary tract excludes other possible causes of symptoms that may not result from the prostate, such as urinary tract infection, neurogenic bladder, urethral stricture, or prostate cancer.
A physical examination, digital rectal examination (DRE), and focused neurologic examination are performed on all patients. The size and consistency of the prostate is noted, even though prostate size, as determined by DRE, does not correlate with severity of symptoms or degree of obstruction. BPH usually results in a smooth, firm, elastic enlargement of the prostate. Induration, if detected, must alert the physician to the possibility of cancer and the need for further evaluation (i.e., prostate-specific antigen (PSA), transrectal ultrasound (TRUS), and biopsy) [7].
A urinalysis to exclude infection or hematuria and serum creatinine measurement to assess renal function are required. Renal insufficiency may be observed in 10 % of patients with prostatism and warrants upper-tract imaging. Patients with renal insufficiency are at an increased risk of developing postoperative complications following surgical intervention for BPH.
Serum PSA is considered optional, but most physicians will include it in the initial evaluation [8].
In some cases of benign prostatic hyperplasia are also observed high levels of PSA, but they are not considered index of malignant progression, but rather are due to the increase in prostate volume, consequently the most production of the antigen itself. PSA, compared with DRE alone, certainly increases the ability to detect CaP, but because there is much overlap between levels seen in BPH and CaP, its use remains controversial (see Screening for CaP) [9].
7.1.5 Pathological Findings
Benign prostatic hyperplasia is a disease characterized by an enlargement (hyperplasia or hypertrophy) of the prostate, more specifically in the prostatic epithelial cells and stromal cells, which leads to the formation of nodules in the periurethral region of the prostate. When these nodules become large enough to compress the urethral canal, causing a partial obstruction of the same, they interfere with normal urinary flow.
The hyperplastic prostate may consist of pure fibroleiomyomatous nodules or of pure glandular or of mixed glandular-atrophic-cystic areas. Frequently, these structures are intermixed. The smooth muscle fiber content of the prostate changes when concomitant chronic inflammatory processes are present. If, after transurethral resection in a recurrent hyperplastic gland, chronic transurethral resection (TUR)-prostatitis has developed, the fibromatous portion increases with little or no nodule formation. Vascularization and cellular content also change.
The prostate is an immunocompetent organ, and it is normally populated by a small number of inflammatory cells (i.e., T and B lymphocytes, macrophages, and mast cells).
Prostate tissue inflammatory patterns have been also widely assessed in patients with BPH. Robert et al. recently characterized the inflammatory infiltrate in a large cohort of patients surgically treated for BPH. Interestingly, 81 % of patients had T-lymphocytes infiltrates (CD-3), 52 % B cells (CD-20), and 82 % macrophage markers (CD-163) in BPH tissues. Thus, T cells represented the major component of the inflammatory infiltrate seen within the prostate gland. Moreover, an increased expression of B lymphocytes and macrophages was reported. These antigen-presenting cells play an important role in the activation of T lymphocytes and in the subsequent onset of an inflammatory state.
Prostatic calcifications are common in men with BPH and their incidence increases with age. Interestingly, calcifications were also seen in 47.2 % of younger men aged <50 years with urological complaints. In some studies [10], the incidence and significance of calcifications in patients with chronic pelvic pain syndrome were evaluated. In these patients, calcifications detected at TRUS were significantly associated with greater inflammation and longer symptoms duration. Other authors found that patients with larger stones were at higher risk of developing LUTS or prostatitis [11]. It could be speculated that in young patients, alterations in the prostatic fluid related to infections or inflammatory diseases might result in prostatic calcifications, which may obstruct intraprostatic ducts and subsequently enhance the inflammatory process. Particularly, calcifications could be the expression of previous infections leading to a chronic inflammatory process, with higher risk of subsequent BPH development over time.
7.1.6 MR Imaging
In the last few years, magnetic resonance imaging (MRI) has been shown to be of great value in the imaging of the pelvis. Its advantages derive from the capability to obtain sections in direct axial, sagittal, and coronal planes and to show greater soft-tissue contrast than any other imaging modality [12]. In fact, CT does not easily provide direct coronal and sagittal images and does not visualize the internal architecture of prostate and seminal vesicles. Transrectal US is widely used as a first-choice exam in the study of prostatic pathology and gives a clear demonstration of the glandular texture. However, its limitation, when compared with MRI, is that it provides only a limited view of the pelvic structures surrounding the prostate and seminal vesicles.
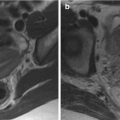
T2-weighted MR imaging is the workhorse of prostate MR imaging. T2-weighted MR images have high spatial resolution and, thus, can clearly differentiate the normal intermediate- to high-signal-intensity peripheral zone (PZ) from the low-signal-intensity central and transition zones in young male subjects. In the aging man, owing to variable extension of the transition zone due to benign prostatic hyperplasia (BNH), the size and signal intensity of the prostate transition zone may vary. BPH itself is a round, well-defined, inhomogeneous area with (variable) intermediate signal intensity and a low-signal-intensity rim that surrounds the expanded transition zone. Because of transition zone expansion, the remainder of the compressed central zone is often indefinable on MR images (Fig. 7.1) [13, 14].
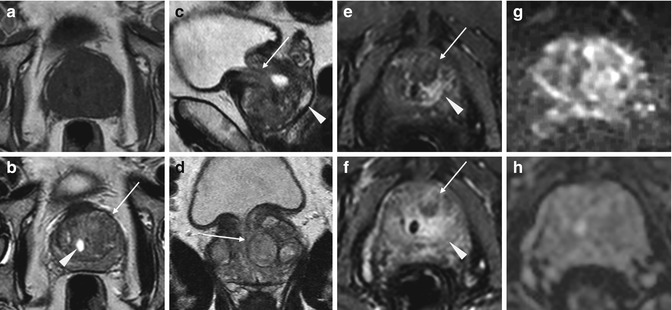

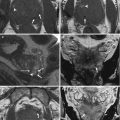
Fig. 7.1
Benign prostatic hyperplasia: signal intensity of adenomas; compression of urethra and peripheral zone. (a) Axial T1WI (TR 519/TE 10) shows an enlargement of prostate gland. (b) Axial T2WI (TR 3072/TE 100) depicts the presence of multiple adenomatous nodules that subvert the normal signal intensity of the transition zone (arrow). These nodules have inhomogeneous signal intensity but well-defined hypointense margins as we can determine their size and morphology. A cystic formation is recognizable in periurethral space on the right side (arrowhead). (c, d) Sagittal (TR 5712/TE 120) and coronal T2WI (TR 3000/TE 100) show very well the increase in the size of the central area, responsible of compression of the urethra (arrow) and of peripheral zone (arrowhead). (e) Early dynamic contrast-enhanced gradient-echo image (TR 4.02/TE 1.91) shows no enhancement of prostatic adenomas (arrow) because of their high fibrous component but depicts an early irregular enhancement in the left side of the central zone (arrowhead). (f) Later dynamic contrast-enhanced gradient-echo image (TR 4.01/TE 1.91) shows a normal progressive enhancement of prostatic adenomas (arrow) and does not show any washout in the left region of the central zone (arrowhead) indicating that it is an inflammation zone. (g, h) DWI (b800) and ADC image does not show significant restricted diffusion in the examined areas
High-spatial-resolution T2-weighted rapid acquisition with refocused echo sequences with a small field of view, performed with endorectal and/or external body phased-array coils, is generally used to depict prostate anatomy. T1-weighted contrast in the prostate is very low. Therefore, it is not possible to appreciate the different anatomic zones on T1-weighted images. Therefore, a suggestion for minimal requirements for multiparametric MR imaging is a combination of T1- and T2-weighted imaging with DW or dynamic contrast-enhanced MR imaging. Owing to the presence of BPH, cancer in central and transition zones is more difficult to discern, BPH may have signal intensity similar to that of prostate cancer on T2-weighted images. However, it has been reported that features such as low-signal-intensity lesions with a wedge shape and a diffuse extension without mass may be reliable signs of benignity. Dynamic contrast-enhanced (DCE) MR imaging consists of a series of fast T1-weighted sequences covering the entire prostate before and after rapid injection (2–4 mL/s) of a bolus of a low-molecular-weight gadolinium chelate. A fast and direct method of characterizing prostatic vascular pharmacokinetic features is high temporal resolution DCE-MRI (<10 s). DCE-MRI consists of a series of axial T1WI gradient echo sequences covering the entire prostate during and after IV bolus injection of gadolinium contrast medium [15]. Diffusion-weighted imaging (DWI) is a powerful clinical tool, as it allows apparent diffusion coefficient (ADC) maps to be calculated, enabling qualitative and quantitative assessment of the prostate. Diffusion-weighted imaging should be acquired in the axial plane with an echo planar imaging sequence employing parallel imaging [16].
The MRI appearance of BPH is characteristic, though no tissue-specific diagnosis of benign disease can be made with certainty. The prostate is usually enlarged but can also be of normal size. On T1WI, the gland has a homogeneous intermediate-low signal intensity with no evidence of intraglandular zonal abnormalities. On T2WI, the appearance is that of a nonhomogeneous texture, mainly in the central gland, with alternating areas of intermediate and high signal (Fig. 7.1). The different signals of the various nodules are related to their relative stromal or epithelial components. The benign central gland is composed of two histologically distinct types of tissue: glandular tissue, which is hyperintense on T2-weighted images (Fig. 7.2), and stromal tissue, which is hypointense and can mimic CG cancer (Fig. 7.3). Like CG cancer, the majority of stromal hyperplastic foci could also demonstrate homogeneously low signal intensity and had ill-defined margins. The majority of glandular tissue foci are homogeneously hyperintense.
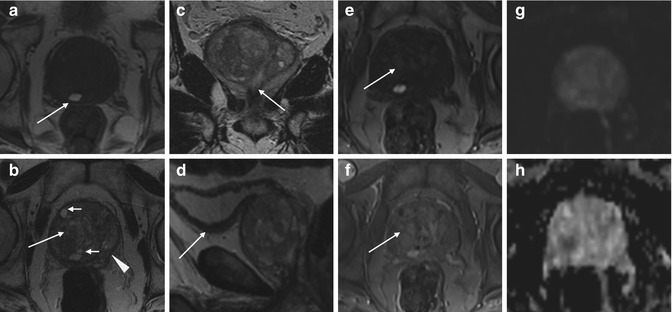
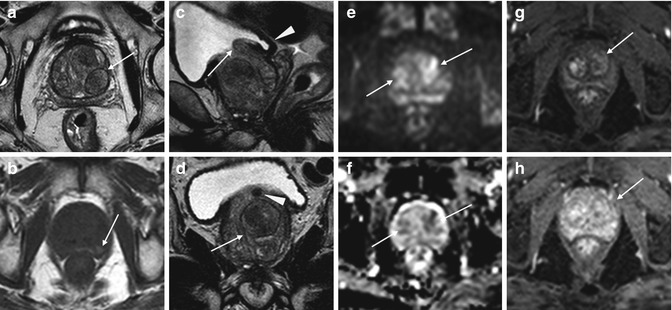

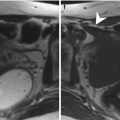
Fig. 7.2
Benign prostatic hyperplasia, normal pattern of adenomas enhancement. (a) Axial T1WI (TR 520/TE 12) shows an enlargement of the prostate gland, and a hyperintense cyst with proteinaceous content is evident in the right posterior region of the peripheral zone (arrow). (b) Axial T2WI (TR 3072/TE 100) shows a heterogeneous signal intensity in the transition zone for the adenomatous changes of the gland (arrow), with compression of the peripheral zone that appears thinned (arrowhead). There are also some small retention cysts of the gland (short arrow). (c) Coronal T2WI (TR 3000/TE 100) shows the compression/deflection of intraprostatic urethra by adenomatous hyperplasia. (d) Sagittal T2WI (TR5000/TE120) in which we can see a diffuse thickening of the bladder (arrow) wall because of chronic outflow obstruction. (e) Early dynamic contrast-enhanced gradient-echo image (TR 4.02/TE 1.91) (30 s) shows a slight and diffuse enhancement of the adenomatous central zone (arrow). (f) Later dynamic contrast-enhanced gradient-echo image (TR 4.02/TE 1.91) shows a greater and progressive enhancement of central gland adenomas without any washout (arrow) (no characteristics of pathology). (g, h) DWI (b800) and ADC images do not show significant restricted diffusion areas in the gland

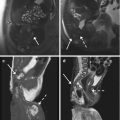
Fig. 7.3
Benign prostatic hyperplasia, diffusion restriction in stromal hyperplastic foci. Presence of post-biopsy hemorrhagic foci. (a) Axial T2WI (TR 3027/TE 100) shows an increase in volume of the transition zone in which context are appreciable (referred to nodules) multiple large hyperplastic nodules, typically surrounded by a hypointense rim of tissue (arrow). (b) Axial T1WI (TR 600/TE 13) shows some hyperintense foci in the peripheral zone attributable to post-biopsy hemorrhagic foci (arrow). (c) Sagittal T2WI (TR 5700/TE 130) shows the imprint of the transition zone of the prostate on bladder floor (arrow). Bladder walls are slightly thickened for detrusorial hypertrophy (arrowhead). (d) Coronal T2WI (TR 3000/TE 100) in which we can see the compression/deflection of intraprostatic urethra by adenomas (arrow). There is also a little calcification in the anterosuperior portion of the transitional region (arrowhead). (e, f) DWI (b800) and ADC show in this case some hyperintense and hypointense areas respectively, a sign of restricted diffusion, corresponding to some adenomatous nodules of the gland (arrows). (g) Early dynamic contrast-enhanced gradient-echo image (TR 3.8/TE 2) (30 s) shows a slight and diffuse enhancement of the adenomatous central zone (arrow). (h) Later dynamic contrast-enhanced gradient-echo image (TR 3.8/TE 2) (1.30 min) shows a progressive enhancement of central gland adenomas (arrow) without any washout (no characteristics of pathology)
Sometimes each individual adenomatous nodule is surrounded by a hypointense rim of tissue (Figs. 7.1 and 7.4). MR images are usually obtained in more than one plane to allow accurate evaluation of prostatic volume. In addition, sagittal images are particularly suited to demonstrate impingement on the base of the bladder and the effect of the adenomatous nodules on the bladder neck and prostatic urethra. Stromal and sclerotic nodules of benign hyperplasia, if located in the peripheral zone where malignancy and inflammation usually occur, can appear the same as prostatitis or carcinoma.
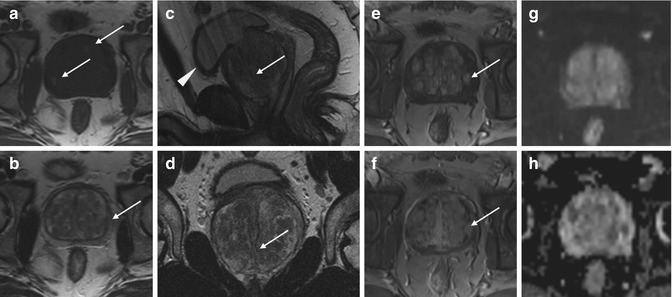

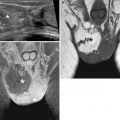
Fig. 7.4
Benign prostatic hyperplasia, normal pattern of adenomas enhancement. (a) Axial T1WI (TR 550/TE 9.3) shows an enlargement of prostate gland, and some slight hyperintense areas compatible with proteinaceous content cysts are evident in the right and posterior region of the peripheral zone (arrows). (b) Axial T2WI (TR 4480/TE 108) shows a heterogeneous signal intensity in the transition zone for the adenomatous changes of the gland, with compression of the peripheral zone which appears thinned (arrow). (c, d) Sagittal T2WI (TR 3000/TE 114) and coronal T2WI (TR 2860/TE 116) show very well the increase in the size of the central area, responsible of compression/deflection of intraprostatic urethra (arrow) and a diffuse thickening of the bladder wall because of chronic outflow obstruction (arrowhead). (e) Early dynamic contrast-enhanced gradient-echo image (TR 12/TE 15.76) shows a slight and diffuse enhancement of the adenomatous central zone (arrow). (f) Later dynamic contrast-enhanced gradient-echo image (TR 12/TE 5.76) shows a greater and progressive enhancement of central gland adenomas without any washout (arrow) (no characteristics of pathology). (g, h) DWI (b800) and ADC images do not show significant restricted diffusion areas in the gland
Dynamic contrast-enhanced MRI in BPH can show accentuation of contrast already in the early phase after contrast administration but more frequently has a more delayed enhancement that increases progressively up to a plateau, reflecting the increased microvessel density in BPH (Figs. 7.2 and 7.5) [13].
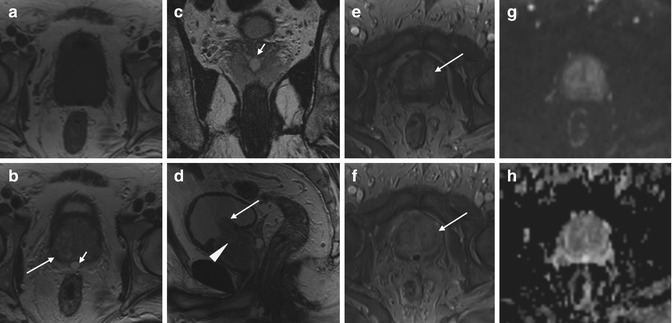

Fig. 7.5
Benign prostatic hyperplasia, normal pattern of adenomas enhancement. Presence of ejaculatory duct cyst. (a) Axial T1WI (TR 550/TE 9.3) shows an enlargement of the prostate. The gland has a homogeneous intermediate-low signal intensity with no evidence of intraglandular zonal abnormalities. (b) Axial T2WI (TR 5130/TE 108) shows a heterogeneous signal intensity in the transition zone for the adenomatous changes of the gland, with compression of the peripheral zone that appears thinned (arrow). There is also an ejaculatory duct cyst of 9 mm (short arrow). (c) Coronal T2WI (TR 3600/TE 116) shows the ejaculatory duct cyst behind the gland (short arrow). (d) Sagittal T2WI (TR3600/TE114) shows the imprint of the transition zone of the prostate on bladder floor (arrow) and the compression/deflection of intraprostatic urethra by adenomatous hyperplasia (arrowhead). (e, f) Early and later dynamic contrast-enhanced gradient-echo images (TR 12/TE 5.7) show a slight and diffuse enhancement of the adenomatous central zone and a greater and progressive enhancement of central gland adenomas without any washout (arrow). (g, h) DWI (b800) and ADC images do not show significant restricted diffusion areas in the gland
Diffusion MR imaging parameters of central gland hyperplasia change according to the presence of stromal (ADC 1.27 × 10−3 mm2/s) or glandular hyperplasia (ADC: 1.73 × 10−3 mm2/s); therefore, it can be misleading to calculate a single diffusion parameter to represent the entire benign central gland. There is a considerable overlap between central gland carcinoma and stromal hyperplastic foci (Fig. 7.4).
7.1.7 Differential Diagnosis
7.1.7.1 Prostate Cancer
Prostate cancer (PC) may be detected by abnormalities on the DRE or an elevated PSA.
PSA, compared with DRE alone, certainly increases the ability to detect PC, but because there is much overlap between levels seen in BPH and PC, its use remains controversial. Conventional MR imaging is generally considered inadequate for evaluating CG cancers because of the heterogeneous T2 signal intensity in the normal transition zone. It has been suggested that homogeneous low T2 signal intensity, ill-defined margins, and lack of a capsule can be useful in the identification of PC. Lenticular shape and invasion of the anterior fibromuscular stroma can help differentiate cancer from benign central gland (CG).
In small studies, CG cancers demonstrated restricted diffusion, with smaller ADCs (0.93–1.37 × 10−3 mm2/s) compared with BPH in the CG (1.34–1.79 × 10−3 mm2/s), but other studies are discordant with these values. Despite a consensus in multiple series of restricted diffusion in CG cancers, there is substantial variation in reported ADCs of benign CG tissue [17]. Often, it is very hard to distinguish this pattern from that of a gland carcinoma central, which however enhances more and wash out faster than benign tissue [13].
7.1.7.2 Prostatitis
A urinary tract infection, which can mimic the irritative symptoms of BPH, can be readily identified by urinalysis and culture; however, a urinary tract infection can also be a complication of BPH (see other chapter).
7.1.7.3 Other Obstructive Conditions
Other obstructive conditions of the lower urinary tract, such as urethral stricture, bladder neck contracture, and bladder stone, must be entertained when evaluating men with presumptive BPH. A history of previous urethral instrumentation, urethritis, or trauma should be elucidated to exclude urethral stricture or bladder neck contracture.
Although irritative voiding complaints are also associated with carcinoma of the bladder, especially carcinoma in situ, the urinalysis usually shows evidence of hematuria. Likewise, patients with neurogenic bladder disorders may have many of the signs and symptoms of BPH, but a history of neurologic disease, stroke, diabetes mellitus, or back injury may be present as well. In addition, examination may show diminished perineal or lower extremity sensation or alterations in rectal sphincter tone or the bulbocavernosus reflex. Simultaneous alterations in bowel function (constipation) might also alert one to the possibility of a neurologic origin.
7.1.8 Treatment
After patients have been evaluated, they should be informed of the various therapeutic options for BPH. It is advisable for patients to consult with their physicians to make an educated decision on the basis of the relative efficacy and side effects of the treatment options.
Specific treatment recommendations can be offered for certain groups of patients. For those with mild symptoms (symptom score 0–7), watchful waiting only is advised.
On the other end of the therapeutic spectrum, absolute surgical indications include refractory urinary retention (failing at least one attempt at catheter removal), recurrent urinary tract infection from BPH, recurrent gross hematuria from BPH, bladder stones from BPH, renal insufficiency from BPH, or large bladder diverticula [18, 19].
A.
Watchful Waiting
Watchful waiting is the appropriate management of men with mild symptom scores.
B.
Medical Therapy
1.
Alpha-blockers—The human prostate and bladder base contains alpha-1-adrenoreceptors, and the prostate shows a contractile response to corresponding agonists. The contractile properties of the prostate and bladder neck seem to be mediated primarily by the subtype alpha-1a receptors. Alpha-blockade has been shown to result in both objective and subjective degrees of improvement in the symptoms and signs of BPH in some patients.
2.
5-Alpha-reductase inhibitors—Finasteride is a 5-alpha-reductase inhibitor that blocks the conversion of testosterone to dihydrotestosterone. This drug affects the epithelial component of the prostate, resulting in a reduction in the size of the gland and improvement in symptoms.
Dutasteride differs from finasteride as it inhibits both isoenzymes of 5-alpha reductase. Similar to finasteride, it reduces serum prostatic-specific antigen and total prostate volume.
3.
Combination therapy—The reduction in risk associated with combination therapy (66 % risk reduction) is significantly greater than that associated with doxazosin or finasteride alone. Patients most likely to benefit from combination therapy are those in whom baseline risk of progression is very high, generally patients with larger glands and higher PSA values.
C.
Get Clinical Tree app for offline access

Conventional Surgical Therapy
1.
Transurethral resection of the prostate—Symptom score and flow rate improvement with transurethral resection of the prostate (TURP) is superior to that of any minimally invasive therapy. Much controversy revolves around possible higher rates of morbidity and mortality associated with TURP in comparison with those of open surgery, but the higher rates observed in one study were probably related to more significant comorbidities in the TURP patients than in the patients undergoing open surgery. Several other studies could not confirm the difference in mortality when results were controlled for age and comorbidities.
2.




Transurethral incision of the prostate—Men with moderate to severe symptoms and a small prostate often have posterior commissure hyperplasia (elevated bladder neck). These patients will often benefit from an incision of the prostate. This procedure is more rapid and less morbid than TURP. Outcomes in well-selected patients are comparable, although a lower rate of retrograde ejaculation with transurethral incision has been reported (25 %).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree