Anatomic location
Symptoms
Pouch of douglas, torus uterinus, USLs, rectovaginal septum
Dyspareunia
Bowel
Dyschezia, catamenial diarrhea, rectal bleeding, constipation, intestinal occlusion
Ureteral stenosis
Urinary obstruction, flank pain
Bladder
Urgency, frequent urination, hematuria
Pleura – lung
Hemophthysis, chest pain, shortness of breath
Brain
Perimenstrual headaches, seizures, subarachnoid hemorrhage
Skin
Catamenial bleeding, tenderness
Infertility may be the presenting complaint of endometriosis, with or without pelvic pain. It is estimated that 30–50 % of women with endometriosis are infertile, and 20 % of infertile women have endometriosis [14]. The pathogenic mechanism is debated. It could be related to repeated episodes of bleedings causing a chronic inflammatory state, which can lead not only to anatomic alterations induced by fibrosis and adhesions, but also to bio-humoral changes [15].
Of the three forms, deep pelvic endometriosis is thought to contribute most often to clinical symptoms. Then, in case of ovarian endometrioma with chronic and severe pelvic pain, associated deep pelvic endometriosis lesions are generally present, often multifocal and with intestinal infiltration [16].
4.1.4 Diagnosis
The lack of specific symptoms makes the diagnosis of endometriosis difficult and, often, very late. Diagnosis is made harder because the first-line imaging study, usually transvaginal or transabdominal ultrasound (US) [17], has a high sensitivity for adnexal lesions but a poor accuracy for other locations, especially for deep pelvic endometriosis, frequently giving false-negative results [17]. Above transabdominal and transvaginal US, several techniques have been used: magnetic resonance (MR) imaging and, in case of suspected bowel endometriosis, transrectal ultrasonography (US), rectal endoscopic sonography, and double barium contrast enema (DBCE). When the urinary system is involved, MR urography and cystography could be employed.
The reference standard for the diagnosis of pelvic endometriosis remains laparoscopy with histological confirmation.
In this chapter, we would highlight the role of magnetic resonance (MR) imaging in the diagnosis of endometriosis. This technique has already been demonstrated as a successful diagnostic tool to investigate the tubo-ovarian endometriosis, with a sensitivity, specificity, and accuracy of 90, 98, and 96 %, respectively [18], but also for deep endometriosis, with a sensitivity and specificity of 90 and 91 %, respectively [19].
4.2 Uterine Adenomyosis
Adenomyosis is a common non-neoplastic uterine disease defined by the presence of ectopic endometrial tissue within the myometrium, due to the invagination of endometrium in the myometrium at a depth of at least 2.5 mm below the basal layer of the endometrium. This process leads to hyperplasia and hypertrophy of the smooth muscle [20].
Adenomyosis can be either focal (one or several foci in the myometrium) or diffuse (numerous foci spread throughout the myometrium), and it is often asymmetric, predominating in the posterior uterine corpus. More controversial is the distinction between superficial (simple thickening of the junctional zone seen on MRI or lesions not extending beyond one third of the depth of the myometrium) and deep forms (penetrating deeper than one third of the myometrium). Although it has been accepted that uterine adenomyosis results from the direct invasion of the endometrium into the myometrium, and in such cases no direct relationship between the adenomyosis and the endometrium is proved histologically. Rather the disease appears to be the result of the invasion of endometrium-like structures (presumably endometriosis) from outside the uterus, disrupting the uterine serosa. This case has been named by some authors as “external” (or “extrinsic”) adenomyosis, connected to lesions of deep pelvic endometriosis [21] extending to the uterine myometrium, frequently sparing the endometrial-myometrial junctional zone. The definition of this form as “external” adenomyosis or DPE involving the uterus is still debated; however, it practically does not affect therapy.
Furthermore, in some cases adenomyosis is present in the myometrium completely isolated from both the endometrium and the serosa. Such a difference would postulate a new hypothesis that adenomyosis is composed of multiple heterogeneous subtypes [22].
4.2.1 Imaging Features of Adenomyosis
4.2.1.1 Ultrasonography
The transvaginal ultrasonography (TVUS) is usually the first-line investigation in cases of suspected adenomyosis. The transabdominal ultrasonography has a poor sensitivity (53–89 %) but a good specificity (97 %). The combination of transabdominal and transvaginal ultrasonography (TVUS) increases the diagnostic accuracy.
The direct signs of adenomyosis are:
1.
Anechoic subendometrial microcysts in the myometrium (2–4 mm) that can be distinguished from vascular images on Doppler sonography because they are not vascularized (pathognomonic sign). This cystic space corresponds to ectopic dilated endometrial glands into the myometrium; if they are hemorrhagic, their content shows greater echogenicity.
2.
Inhomogeneous appearance of the myometrium with hyperechoic linear striations (correlated to its hypertrophy).
3.
Small hyperechoic subendometrial nodules, pseudonodular hypoechoic zones with indistinct contours and no mass effect on the endometrium.
4.
A poorly defined or thickened endometrial-myometrial junctional zone (JZ).
The indirect signs of adenomyosis are:
1.
The uterus is enlarged, rounded with regular contours.
2.
Asymmetric thickening of the myometrium, especially in case of focal adenomyosis, results from reactive hyperplasia and hypertrophy of the smooth muscle fibers around the ectopic endometrial glands.
3.
Linear pattern of vascularization on Doppler sonography, crossing the myometrium within the adenomyosic lesion (opposed to the leiomyoma).
4.
A poorly defined or thickened endometrial-myometrial junctional zone (JZ).
4.2.1.2 Magnetic Resonance Imaging (MRI)
Pelvic MRI is the second-line investigation, and it offers the best performance when there is any doubt over diagnosis and in terms of looking for any pathology associated with adenomyosis.
Pelvic MRI is superior to TVUS in terms of sensibility and specificity for both focal and diffuse adenomyosis.
The MRI signs of adenomyosis can be classified into direct and indirect.
The direct MRI signs of adenomyosis are:
1.
On T2-weighted images, typical adenomyosis appears as an ill-demarcated low-signal-intensity area (Fig. 4.1a, c, e, f), owing to abundant smooth muscle proliferation, with or without punctuate high-signal-intensity foci scattered throughout the lesion or high-signal-intensity linear striations extending from the endometrium. Hyperintense foci could be present on T1-weighted images, more evident with fat saturation, corresponding to small areas of hemorrhage (Fig. 4.1b, d).
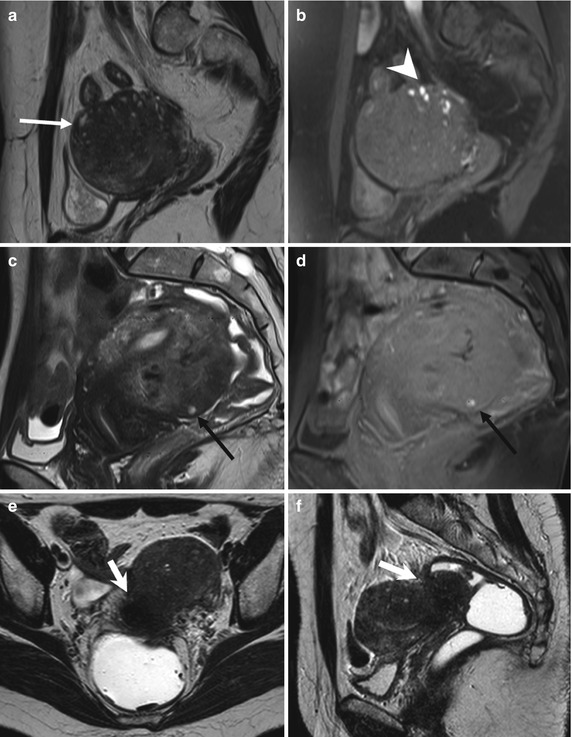

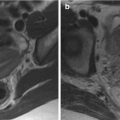
Fig. 4.1
Diffuse adenomyosis. Sagittal (a, c, f) and axial (e) T2-weighted FSE images. Sagittal T1-weighted FS-GRE image (b, d). a (TR 4670 TE 94), b (TR 369, TE 10). The uterus is markedly enlarged, especially in the posterior portion, with regular contours and a thickened and poorly recognizable endometrial-myometrial junctional zone (JZ). On T2-weighted images this low-signal-intensity area contains multiple small foci hyperintense (a: arrow), which represent ectopic endometrial glands and small subendometrial cysts. The high-signal-intensity spots on T1-weighted fat-saturated image (b: arrowhead) correspond to multiple area of hemorrhage within the ectopic endometrial tissue. c (TR 3870 TE 100), d (TR 369, TE 10). The uterus is retroflexed and markedly enlarged in its posterior portion, with hyperintense spots in both T1- and T2-weighted images (black arrow). These findings are consistent with adenomyosis. e (TR 4530 TE 90), f (TR 5390 TE 113). Also in this case the uterus is enlarged, and the JZ is thickened. Moreover a heterogeneous hypointense nodule on T2-weighted images is recognizable in the pouch of Douglas (short arrows), suggestive of deep pelvic endometriosis (DPE) location. Obliteration of the fat tissue plan and invasion of both the posterior uterine surface and anterior rectosigmoid wall are present
2.
An important sign of adenomyosis is the presence of subendometrial microcysts, related to the presence of endometrial glands within the myometrium. MRI reveals round cystic foci varying from 2 to 7 mm in diameter, hyperintense on T2-weighted sequence, embedded within the myometrium, and usually located within the JZ. Sometimes, at the end of the menstrual period, hemorrhagic foci within cystic cavities are recognizable and appear as high-signal-intensity spot on T1-weighted images owing to the T1-shortening effects of methemoglobin. This hemorrhagic content is not found routinely because adenomyotic endometrium, like the basalis endometrium, seldom responds to hormonal stimuli with cyclic changes, but it is less common than in endometriosis. Susceptibility-weighted imaging (gradient echo – GRE sequences) is sensitive for old hemorrhagic foci, which appear as spotty signal voids owing to the T2*-shortening effects of accumulated hemosiderin.
At diffusion-weighted images, adenomyosis has low to intermediate signal intensity, a finding consistent with its benign, non-neoplastic nature. Because adenomyosis may show various degrees of enhancement after administration of contrast medium, dynamic study does not contribute to diagnostic accuracy. However, additional MR angiographic GRE sequences are recommended in patients with adenomyosis in whom uterine artery embolization is planned.
The indirect signs of adenomyosis are secondary to the reactive hyperplasia of the myometrium provoked by endometrial invasion and include:
1.
Globular aspect of the enlarged uterus with regular contours.
2.
Asymmetric thickening of the myometrial walls (more common of the posterior wall).
3.
Thickening of endometrial-myometrial junctional zone (JZ: Fig. 4.1). Generally, a JZ thickness of greater than 12 mm is the accepted criterion in establishing the presence of adenomyosis (JZ ≥12 mm). Also the greatest JZ thickness to total myometrium ratio >40–50 % is considered [23]. However, adenomyosis can be excluded if the JZ thickness is 8 mm or less [24]. Anyway, from 20 to 30 % of patients will not have a visible or measurable endometrial-myometrial junctional zone during their reproductive cycle [25].
4.2.2 Atypical Morphologic Appearances of Adenomyosis
4.2.2.1 Adenomyoma
Adenomyoma is an atypical morphologic appearance of adenomyosis and is rarer than both focal and diffuse adenomyosis. It is composed of a focal consolidation of adenomyotic glands and appears as a poorly defined nodular lesion with extensive muscular response (Fig. 4.2a, b). It may be intramyometrial, subserosal, and possibly even intracavitary. Like adenomyosis, adenomyoma usually shows heterogeneous low signal intensity on T2-weighted images and may be indistinguishable from degenerated leiomyomas and from aggressive uterine neoplasms such as uterine sarcomas. Relatively low signal intensity at diffusion-weighted imaging with high ADC value is suggestive of its benign nature.
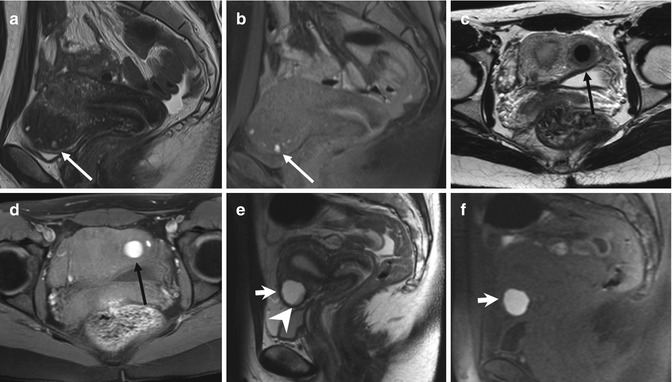

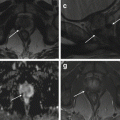
Fig. 4.2
Focal adenomyosis (a, b). Sagittal T2-weighted (a: TR 4070, TE 94) and FS-T1-weighted (b: TR 629, TE 10) images. The uterus is anteverted. A focal myometrial thickening is recognizable on the anterior uterine wall (arrows). This area is heterogeneously hypointense on T2-weighted image, with hyperintense foci on both T1- and T2-weighted sequences, findings consistent with focal uterine adenomyosis. Adenomyotic cysts. Two cases. (c, d) Axial T2-weighted FSE MR image (c: TR 3252, TE 100) and axial T1-weighted fat-suppressed image (d: TR 629, TE 10) show a rounded well-defined intramyometrial lesion (black arrow), located near the left uterine horn. This lesion shows a very low signal intensity on T2-weighted sequence (c) and high signal intensity on T1-weighted image (d), related to its hemorrhagic content. (e, f) Sagittal T2-weighted FSE MR image (e: TR 4530, TE 90) shows another adenomyotic cyst, located in the subserosal layer of the anterior uterine wall, with inhomogeneous high signal intensity content (short arrow) and contextual-dependent fluid-fluid level (arrowhead). This cyst retains high signal intensity on T1-weighted fat-suppressed MR image (f: TR 328, TE 11), due to its hemorrhagic content
4.2.2.2 Adenomyotic Cyst
Adenomyotic cyst (cystic adenomyosis) is a rare variation of adenomyosis that appears as an intramyometrial endometrioma-like lesion, hyperintense on T1-weighted images, surrounded by adenomyotic tissue with low signal intensity on T2-weighted images (Fig. 4.2c–f). A subserosal adenomyotic cyst may mimic an ovarian tumor. The finding of continuity with the myometrium is suggestive of its uterine origin [26].
4.2.3 Malignant Transformation of Adenomyotic Lesions
Malignant transformation of adenomyosis is quite rare and may manifest as a predominantly intramyometrial mass. Imaging findings of an adenomyotic cyst with malignant transformation are similar to those of an endometrioma with malignant transformation. High-signal-intensity hemorrhagic fluid in the adenomyotic cyst on T1-weighted images may mask the enhancement of malignant mural nodules; therefore, contrast-enhanced subtraction imaging may be useful for detection of malignant transformation. Dynamic contrast-enhanced imaging may have greater accuracy than T2-weighted imaging when adenomyosis and endometrial cancer coexist [27]. Diffusion-weighted imaging can demonstrate the malignant foci as areas of high signal intensity.
4.2.4 Differential Diagnosis
Various benign and malignant conditions may mimic adenomyosis: physiologic myometrial contraction, myometrial involvement by deep pelvic endometriosis, leiomyomas, low-grade endometrial stromal sarcoma, and myometrial metastases.
Transient myometrial contraction is a physiologic phenomenon that may mimic focal adenomyosis because it determines focal pseudo-thickening of the junctional zone. This aspect usually disappears on subsequent images or at cine MR images, instead of focal adenomyosis that persists.
Myometrial contractions in the pregnant uterus are commonly seen and usually do not represent a diagnostic dilemma.
To differentiate physiologic myometrial contractions from focal adenomyosis, rapid T2-weighted sequences can be repeated and should be correlated with other views. Useful to eliminate physiologic uterine contractions is the injection of an antiperistaltic drug, if no contraindications are present.
4.3 Ovarian Endometriosis
The most common location of endometriosis is the ovary, where small and shallow endometrial implants develop, leading to adjacent para-ovarian scarring and adhesions. An ovarian enlargement could be present, attributable to repeated episodes of hemorrhage within a deep implant, resulting in endometriosic cysts, also defined as endometriomas (multiloculated cystic lesions). They may completely replace normal ovarian tissue. From the anatomo-pathological point of view, endometriosic cyst walls are generally thick and fibrotic with common areas of discoloration and dense fibrous adhesions. The cyst lining can vary from smooth and pale to shaggy and brown. Their contents can be watery or, more typically, composed of thick, dark, degenerated blood products, depending on the presence and dating of bleeding. This appearance is called “chocolate cyst.”
4.3.1 Ultrasonography
Pelvic ultrasound (especially TVUS) is the method of choice to identify endometriomas, defined as benign ovarian neoplasms persisting after 3 months. They typically appear as multilocular cysts with diffuse low-level internal echoes and hyperechoic foci in their walls (Fig. 4.3c). Internal thin or thick septations may be present. However, unilocular cysts were found in 43 % of endometriomas. The echogenic wall foci differ from true wall nodules because they are more echogenic and smaller than malignant wall nodules. Although the pathological basis of their formation has not been established, it is postulated that they form as cholesterol deposits accumulating in the endometrioma’s wall.
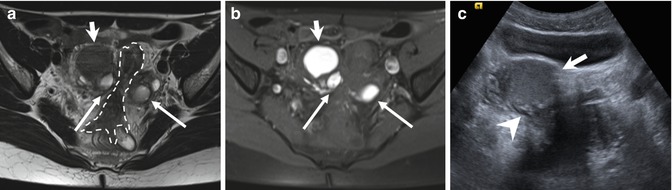

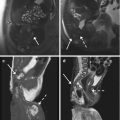
Fig. 4.3
“Kissing ovaries” and bowel adhesions due to endometriosis. Axial T2-weighted FSE MR image (a: TR 4070, TE 94) shows small bilateral ovarian cysts of intermediate signal intensity (arrows) and one right-sided ovarian cyst (short arrow) with marked “T2-shading effect” (low signal intensity). Correspondent axial T1-weighted fat-suppressed MR image (b: TR 351, TE 10) shows bilateral T1-hyperintense endometriomas (arrows and short arrow), confirming the hemorrhagic nature of the cystic content. Adhesions between bilateral endometriomas bring the ovaries closer to the midline (“kissing ovaries” sign). Furthermore there is tight contact with obliteration of the fat tissue plane between ovarian masses and the interposed sigmoid colon (a: dashed line), due to adhesions related to endometriosis. Transverse transabdominal sonogram (c) demonstrates the right-sided endometrioma (short arrow) with diffuse low-level internal echoes and focal wall hyperechoic nodularities (arrowhead)
The endometriomas vary between 30 and 59 mm in maximum diameter in 81 % of cases. The positive predictive value of sonography to predict ovarian endometriosis was evaluated at 75 % when criteria such as diffuse low-level internal echoes and absent neoplastic features were used [28]. The color Doppler imaging shows the absent flow within the lesion. The presence of hyperechoic foci alone at the surface of the ovary is not a sign for endometriosis.
Differential diagnosis includes ovarian functional cysts, mature cystic teratoma, cystadenoma, fibroma, tubo-ovarian abscess, and ovarian carcinoma. The functional cysts, such as corpus luteum or hemorrhagic follicular cysts, will disappear or decrease in size at short-time follow-up. Ovarian cancer can be difficult to exclude if wall irregularities or nodules are present; absence of color Doppler flux within the cyst helps to confirm the benign nature of the lesion. Whenever sonographic features of ovarian masses are uncertain or indeterminate, MRI is the imaging modality of choice to rule out malignancy.
4.3.2 MRI Findings
Superficial peritoneal ovarian endometriosis, not visible at TVUS, can be only rarely detected by MRI, using fat-suppression techniques, as hemorrhagic superficial foci [2]. Their signal intensity is quite variable [3].
Instead endometriomas have typical MRI features, and this technique has a sensitivity and specificity reported of 90 and 98 %, respectively, in the definitive diagnosis [29]. Endometrioma appears as a cystic mass with internal high signal intensity on T1-weighted images, also with fat suppression (Figs. 4.3, 4.4, and 4.5). The walls are usually thickened, and, frequently, loss of the interface between lesion and adjacent organs is present (Fig. 4.3).
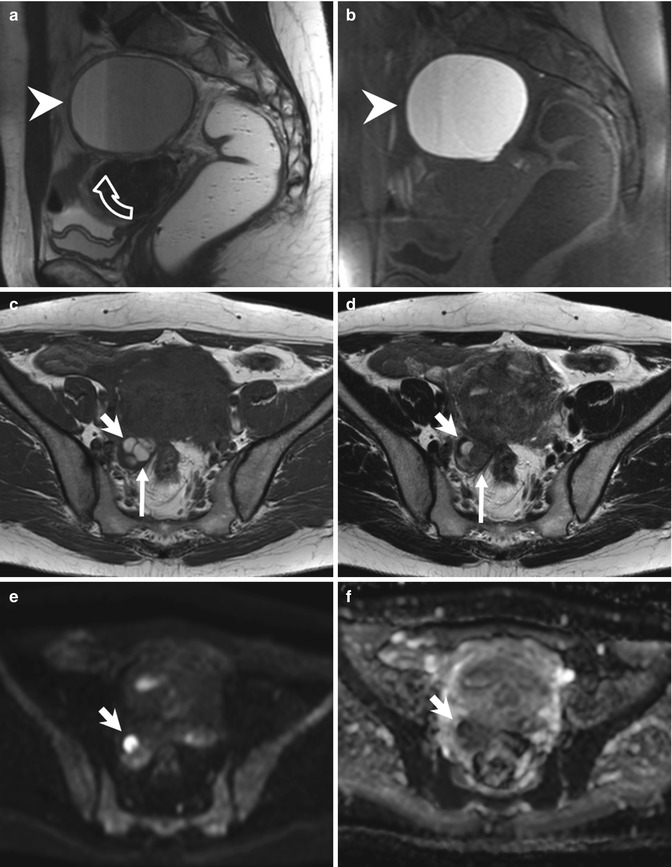
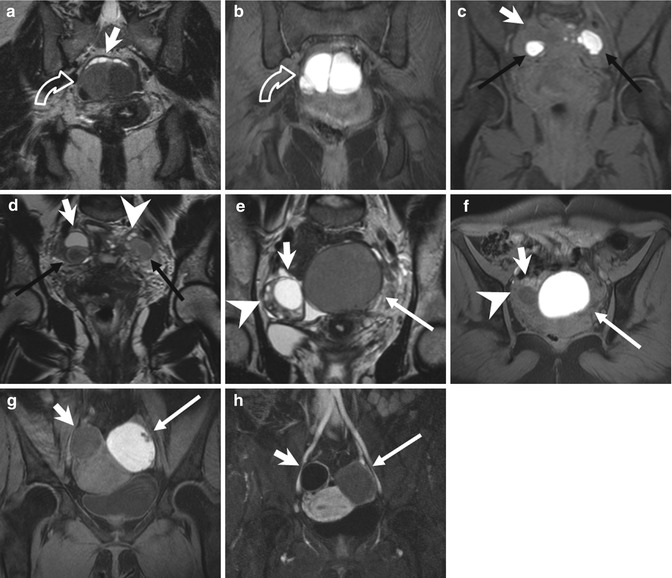

Fig. 4.4
Large endometrioma (a, b). Sagittal T2- (a: TR 4530, TE 90) and T1-FS-weighted (b: TR 328, TE 11) images show a large adnexal cystic lesion (arrowheads) with fluid-fluid level (a: curved arrow). The dependent component appears hyperintense on T1-weighted image with fat suppression (b) and shows a marked T2-shading effect (hypointensity on T2-weighted image). Small endometriomas (c–f). Axial T1-weighted FSE MR image (c: TR 640, TE 10) shows multiple small T1-hyperintense cystic lesions in the right ovary. Axial T2-weighted FSE MR image (d: TR 3652, TE 100) at the same level shows marked T2 shading (hypointensity) in the posteromedial cystic lesion of the right adnexa (long arrows), instead of the anterolateral cyst (short arrows), hyperintense on both T1- and T2-weighted images. Furthermore only the anterolateral cyst (short arrows) presents a restricted diffusion, seen in almost half of endometriomas: in the correspondent diffusion-weighted MR image obtained with b value of 800 s/mm2 (e: TR 7198, TE 69), it shows high signal intensity (short arrow) with low signal intensity on the ADC map (f: TR 7198, TE 69)

Fig. 4.5
Endometriomas (a, b) Coronal T2-weighted FSE (a: TR 5390, TE 113) and fat-suppressed T1-weighted (b: TR 440, TE 4.9) FSE MR images show multiloculated adnexal cystic mass with a “coffee bean” appearance (curved arrow). These cystic formations present high signal intensity on T1-weighted fat-suppressed MR image (b) and marked “T2-shading effect” with consequent lower signal intensity than the adjacent functional ovarian follicles on T2-weighted image (a: short arrow). Bilateral endometriomas (c, d). Coronal fat-suppressed T1-weighted FSE image (c: TR 597, TE 11) and T2-weighted FSE image (d: TR 3520, TE 89). Bilateral endometriomas (black arrows), hyperintense on T1-weighted sequence, present the typical “shading sign” on the T2-weighted image (d: black arrows). Furthermore a hypointense functional cyst (short arrow) is present in the right adnexa and multiple ovarian follicles in the left (arrowhead), both hypointense on T1- and hyperintense on the T2-weighted images. (e–h) Endometrioma of the left ovary. Coronal T2-weighted FSE (e: TR 5390, TE 113), axial T1- (f: TR 493, TE 4.9) and coronal T1-weighted with fat suppression (g: TR 440, TE 4.9) MR images show the two ovaries adjacent to the midline (the “kissing ovaries sign”). A functional cyst (short arrow) and multiple ovarian follicles (arrowhead) are recognizable in the right ovary, both hyperintense on T2- and hypointense on T1-weighted images. In the left ovary an endometrioma presents some parietal nodules (g: arrow), feature suspected for malignant degeneration. The coronal contrast-enhanced T1-weighted fat-suppressed MR image (h: TR 3.7, TE 1.3) demonstrates no contrast uptake within the parietal nodules (arrow), ruling out the suspect of malignant degeneration
Use of chemically selective T1-weighted fat-suppressed sequences is mandatory to visualize smaller endometriomas and to differentiate endometriomas from mature cystic teratomas (hypointense after fat saturation, due to their fat content).
Gradual decrease of signal intensity at T2-weighted image has been described as “T2-shading” sign and is due to chronic bleeding with high concentrations of iron and protein inside the endometriomas (Figs. 4.4a, d and 4.5a, d, e). Adnexal mass with high signal intensity on T1-weighted fat-saturated sequences and signal intensity lower than that of simple fluid on T2-weighted images helps to establish a diagnosis of endometrioma, with a specificity greater than 90 %.
The differential diagnosis with functional hemorrhagic cysts is not always simple. The functional cysts do not demonstrate (or demonstrate less) T2-shading sign and disappear at follow-up imaging. Bilaterality and multifocality of adnexal lesions, along the other characteristics above discussed, can help establish the diagnosis of endometrioma. Bilateral endometriomas occur in more than 50 % of cases, often associated with interovarian adhesions, described as “kissing ovaries” sign (Figs. 4.3 and 4.5e–h). When atypical features of endometriomas are present, such as localized wall thickening, the use of intravenous contrast media is mandatory: the absent enhancement confirms the benign nature of the disease.
At contrast-enhanced T1 sequences (Fig. 4.5h), the peripheral hypointense rim of the endometrioma, representing the thick fibrous capsule, usually shows intense enhancement. Instead the enhancement of solid nodules within a hemorrhagic ovarian cyst has been described in case of ovarian cancer arising within endometrioma [30].
Diffusion-weighted imaging (DWI) with quantitative assessment of apparent diffusion coefficient values (ADC) has often been incorporated into pelvic MR imaging protocols (Fig. 4.4e, f), even though the presence of restricted diffusion and low ADC value within an adnexal lesion does not have a high positive predictive value or specificity for the diagnosis of ovarian malignancy. Benign hemorrhagic ovarian cysts, endometriomas, and solid endometrial implants, as well as benign mature cystic teratomas, also can demonstrate restricted diffusion [31–33].
During pregnancy, increased progesterone levels promote hypertrophy of the endometrial stromal cells and formation of the vascular decidual lining of the uterus. Endometrial stromal cells within endometriomas may also respond to hormonal changes forming vascularized mural nodules. Decidualized endometriosis can mimic ovarian cancer at US and MR imaging. An MR imaging feature specific for decidualized endometriosis is the T2 hyperintensity of the mural nodules, isointense to the thickened decidualized endometrium, and with a broad base. After the end of the pregnancy, decidualized endometriosis has been reported to either resolve or regress to uncomplicated endometriomas (Fig. 4.6) [34].
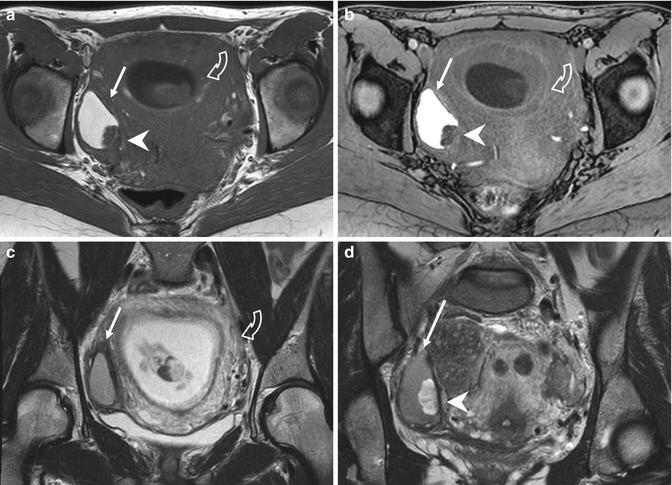

Fig. 4.6
Endometrioma with mural nodule due to decidual reaction of pregnancy (a–d). Axial T1-weighted (a: TR 642, TE 10), FS-T1-weighted (b:TR 5,8 TE 2,8), and T2-weighted images (c, d: TR 4251, TE 100) of a pregnant woman show an enlarged gravidic uterus (curved arrows due to decidual reaction due to pregnancy) and a hyperintense cystic lesion (arrows) in the right ovary. In the posterior part of this cystic formation, a mural nodule (arrowheads) is visible, hypointense on the T1-weighted images, and hyperintense on the T2-weighted sequence. It is a decidualized endometriosis location, related to the hypertrophy of the endometrial stromal cells in the endometrioma. It was due to the increased progesterone levels during pregnancy, and it completely regressed on the MRI images obtained 6 weeks postpartum, when only the endometrioma remained visible
4.3.3 Malignancies Arising in Endometriomas
Women with endometriosis are at risk for developing both clear cell and endometrioid subtypes of epithelial ovarian cancer. An estimated 2.5 % of women with endometriosis develop an ovarian cancer that usually manifests at an earlier stage, with a lower grade and a better prognosis than ovarian malignancies in women with no endometriosis [33, 35].
Endometriosis is one of several benign causes of an abnormal cancer antigen 125 (CA-125) level; thus, an elevated biomarker value alone is not specific for endometriosis-associated ovarian cancer.
The MR imaging features suggestive of malignant endometriomas are the increase in size and, more specific, the development of enhancing mural nodules.
Dynamic subtraction MR imaging is useful in depicting small contrast-enhanced nodules within the hyperintense endometrioma on T1-weighted images. Normal adjacent ovarian parenchyma, intracystic coagulate, inflammation, and decidual change of the endometrium in an endometrioma during pregnancy should be differentiated from malignant transformation. The adjacent ovarian parenchyma may be mistaken for a contrast-enhanced solid malignant component in an endometrioma. An extracystic crescent-shaped portion, which may contain follicles, is the characteristic finding in such cases [33, 35].
4.3.4 Endometriosis of the Fallopian Tubes
Endometriosis is a frequent cause of dilated fallopian tubes; 30 % of women with endometriosis show tubal involvement at laparoscopy. The fallopian tubes are involved by endometrial implants in 6 % and by adhesions in 24 % of cases.
Tubal endometriosis can be divided into two forms, on the basis of implant location: the serosal/subserosal and the intraluminal forms. Both types of tubal endometriosis can be either unilateral or bilateral. The most common serosal or subserosal endometriosis is characterized by implantation of endometrial tissue on the peritoneal surface of the fallopian tubes. Recurrent hemorrhages within the serosal implants presumably result in fibrosis and scarring, leading to peritubal adhesions, obstruction of the tube, and hydrosalpinx.
The intraluminal endometriosis is less common and involves ectopic implantation of endometrium on the mucosal surface of the tube lumen. Cyclic hemorrhage of the implants can cause distention of the fallopian tube with blood, resulting in a hematosalpinx. Hematosalpinx has been reported to be one of the indicators of pelvic endometriosis, and it may be the only imaging finding indicative of endometriosis. However, hematosalpinx is not specific for endometriosis and may have other causes, such as tubal pregnancy, tumors, and tubal torsion. So an accurate assessment of the patient’s history is important.
Hematosalpinx appears on MRI as a hyperintense distention of the fallopian tube on fat-saturated T1- and T2-weighted images (Fig. 4.7). Low T2 signal intensity (T2-shading sign), often seen in endometriomas, is not characteristic of tubal endometriosis, maybe because tubal dilation is most commonly secondary to serosal/subserosal endometrial implants [32, 36, 37].
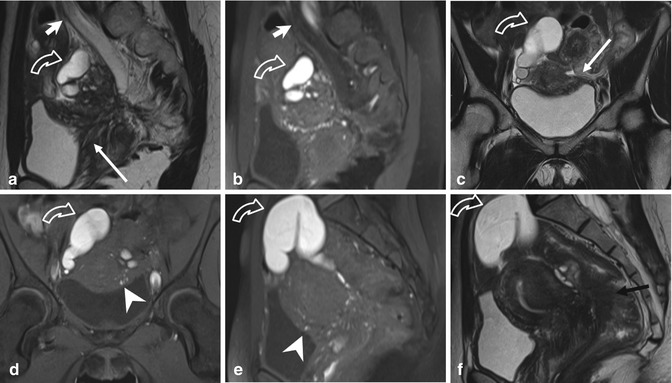

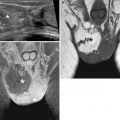
Fig. 4.7




Extensive deeply infiltrating endometriosis involving the urinary system. Sagittal (a, f) and coronal (c) T2-weighted MR images (TR 4670, TE 94). Sagittal (b, e) and coronal (d) T1-weighted fat-suppressed images (TR 369, TE 10). A deep pelvic lesion involves both the anterior and the posterior pelvic compartment; it is located over the bladder dome (a, c: arrows) with obliteration of the vesicouterine pouch (extrinsic bladder endometriosis). Also the distal portion of the left ureter is encased by the endometriosic lesion, with an upstream ureteral dilatation (a, b: short arrows). Intralesional small hyperintense foci on T1-weighted images (d, e: arrowheads) represent microhemorrhages in the ectopic endometrial glands. Bilateral hematosalpinx (tubal endometriosis: curved arrows), more evident on the right fallopian tube, coexists. Also obliteration of the pouch of Douglas and infiltration of the anterior rectal wall (f: black arrow) are present
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree