(1)
Department of Nuclear Medicine, Kuwait University, Safat, Kuwait
15.1 Ionizing Radiation
15.2.1 Direct Effect
15.2.2 Indirect Effect
15.5.1 Early Radiation Effects
15.5.2 Delayed Radiation Effects
Abstract
The main tool in nuclear medicine is ionizing radiation; therefore, it is important for its users to be familiar with its biological effects and its pathophysiological basis. Ionization is the process of ion production by ejection of electrons from atoms and molecules after exposure to high temperature, electrical discharges, or electromagnetic and nuclear radiation. Ionizing radiation is subdivided into electromagnetic radiation (X-rays and gamma rays) and particulate radiation including neutrons and charged particles (alpha and beta particles).
15.1 Ionizing Radiation
The main tool in nuclear medicine is ionizing radiation; therefore, it is important for its users to be familiar with its biological effects and its pathophysiological basis. Ionization is the process of ion production by ejection of electrons from atoms and molecules after exposure to high temperature, electrical discharges, or electromagnetic and nuclear radiation. Ionizing radiation is subdivided into electromagnetic radiation (X-rays and gamma rays) and particulate radiation including neutrons and charged particles (alpha and beta particles).
Exposure to ionizing radiation comes from several natural and man-made sources (Table 15.1). The nuclear medicine professional should be able to provide information to the patient and the public about the radiation risks from these sources and to provide a comparison of exposure from medical procedures to natural sources. Biological effects of ionizing radiation depend on several factors that make them variable and inconsistent. The effects are classified based on their nature and timing after exposure into early or delayed, somatic or hereditary, and stochastic or deterministic (Fig. 15.1). Stochastic effects refer to random and unpredictable effects usually following chronic exposure to low-dose radiation. Hereditary effects and carcinogenesis following diagnostic imaging is of a stochastic nature.
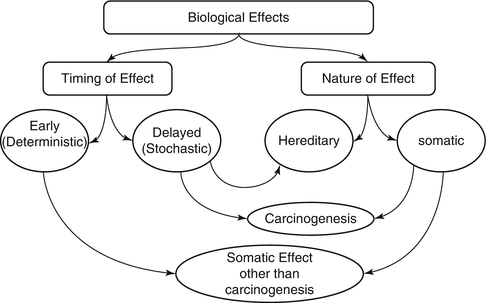
Table 15.1
Sources of ionizing radiation
Natural sources | Man-made sources |
|---|---|
External radiation | Medical |
Cosmic rays | Occupational |
Terrestrial radiation (radioactive material in rocks, such as potassium-40) | Nuclear power |
Nuclear explosions | |
Nuclear accidents | |
Internal radiation | |
Inhalation (radon gas) | |
Ingestion |

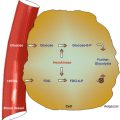
Fig. 15.1
The various biological effects of ionizing radiation. The effects can be classified into early or deterministic, which have a threshold, and delayed or stochastic, with no threshold. Effects are also classified into somatic and hereditary. The somatic include early and delayed effects (cancer)
Deterministic (non-stochastic) effects are nonrandom and have a highly predictable response to radiation. There is a threshold of radiation dose after which the response is dose related. Some of the known deterministic effects are radiation-induced lung fibrosis and cataract.
15.2 Mechanisms of Radiation Effects
Ionizing radiation exerts its effects on biological targets through two major mechanisms [1, 2], direct and indirect (Fig. 15.2).
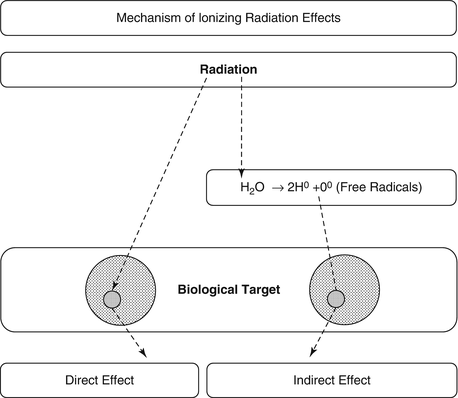

Fig. 15.2
The two mechanisms of ionizing radiation effects on biological tissue, the direct, or target, mechanism and the indirect, through production of free radicals that consequently cause damage
15.2.1 Direct Effect
The direct effect theory or target theory proposes that ionizing radiation acts by direct hits on target atoms. All atoms or molecules within the cells, such as enzymatic and structural proteins and RNA, are vulnerable to radiation injury. DNA, however, is the principal target, in which ionizing radiation produces single- or double-stranded chromosomal breaks.
15.2.2 Indirect Effect
The direct mechanism theory was found to be inadequate in explaining cellular radiation injuries. The indirect theory proposes that ionizing radiation exerts its effect via radiolysis of cellular water, forming free radicals. These free radicals interact with atoms and molecules within the cells, particularly DNA, to produce chemical modifications and consequently harmful effects. When X-rays interact with water, two types of free radicals are formed:
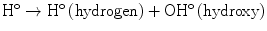
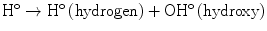
The presence of an excess of oxygen during irradiation of cells allows the formation of additional free radicals:
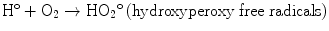
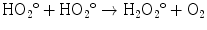
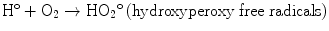
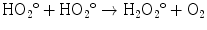
It is worth noting that antioxidants block hydroxyperoxy free radical combination into the highly unstable hydrogen peroxide.
It has been estimated that about two thirds of biological damage caused by low linear energy transfer (LET) radiation is due to indirect action [3]. Biological damage by high LET is primarily, by direct ionization action. Figure 15.3 illustrates how radiation leads to tissue damage.
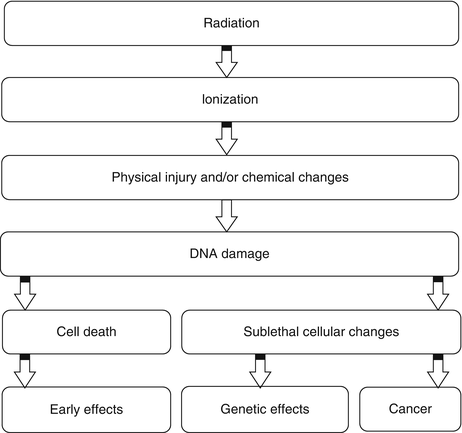

Fig. 15.3
Intracellular changes induced by ionizing radiation that lead to cell damage
Radiation effects have been observed in extents beyond that explained by effects exerted on directly irradiated cells. Cells in temporal or spatial distance from the initial radiation insult have been shown to have delayed effects of radiation. Two phenomenons are described: the bystander effect and genomic instability.
15.2.2.1 Bystander Effect
The cells in the vicinity of irradiated cells show effects that cannot be attributed to targeting by ionizing radiation tracks. Additionally, when cells are irradiated and later transferred to another medium, the cells in proximity in the new medium exhibit DNA damage, mutation, and carcinogenesis. Through cell-to-cell interaction, the directly irradiated cells communicate with adjacent cells and spread the effect of radiation to a larger number of cells. The mechanism is not clearly understood; however, gap junctional intercellular communication [4] or release of soluble factors (such as cytokines) from irradiated cells is proposed. The bystander effect has been mainly described for densely ionizing radiation (such as alpha particles) but also is seen in low LET radiation (such as gamma or X-rays).
15.2.2.2 Genomic Instability
Maximal radiation-induced genetic damage is formed shortly (minutes to hours) after radiation exposure. Nevertheless, it has been observed that not only the irradiated cells but also descendents may show delayed effects. Cells that sustain nonlethal DNA damage show increased mutation rate in descendent cells several generations after the initial exposure. Delayed effects include delayed reproductive death up to six generations following the primary insult.
15.3 Factors Affecting Radiation Hazards
Radiation injury can be modified by factors related to the ionizing radiation and the target tissue.
15.3.1 Factors Related to Ionizing Radiation
Certain factors related to radiation itself determine the various effects for the same radiation dose to biological organs.
15.3.1.1 Type of Radiation
Various types of radiation differ in penetrability based on LET, which expresses energy loss per unit distance traveled (kiloelectron volts per micrometer). This value is high for alpha particles, lower for beta particles, and even less for gamma rays and X-rays. Thus, alpha particles penetrate a short distance but induce heavy damage, and beta particles travel a longer distance but much shorter than gamma rays.
15.3.1.2 Mode of Administration
The radiation dose is obviously an important factor. In addition, a single dose of radiation causes more damage than the same dose being divided (fractionated). Collectively these two factors are expressed as dose per fraction.
15.3.1.3 Dose Rate
Dose rate expresses the time for which dose is administered. The longer the duration for the same total dose, the better the chance of cellular repair and the smaller the damage.
15.3.2 Factors Related to Biological Target
Certain properties of tissues and cells can significantly modify the biological effects of ionizing radiation.
15.3.2.1 Radiosensitivity
Although all cells can be affected by ionizing radiation, normal cells and their tumors vary in their sensitivity to radiation. Slowly and rapidly growing cells have different radiosensitivity in relation to their movement through the cell cycle. Radiosensitivity varies with the rate of mitosis and cellular maturity. Blood-forming cells are very sensitive to radiation, while neurons, muscle, and parathyroid cells are highly radioresistant. Within a given cell, the nucleus in general is relatively more radiosensitive than the cytoplasm.
15.3.2.2 Repair Capacity of Cells
Some cells are known to have a higher capacity than others to repair the damage caused by ionizing radiation; consequently, the biological effects of the same radiation dose are different. Significant repair is known to occur quickly, within 3 h.
15.3.2.3 Cell Cycle Phase
All phases of the cell cycle can be affected by ionizing radiation. The radiosensitivity of a given cell varies from one cell cycle phase to another. Overall, sensitivity appears to be greatest in G2 phase; irradiation during this phase retards the onset of cell division. Irradiation during mitosis induces chromosomal aberrations, i.e., breaks, deletions, translocations, and others. The sensitivity of a given cell cycle phase also differs from one cell type to another and by alteration of radiation injury [3]. For example, the reproductive cells are most sensitive during the M phase, while damage to DNA synthesis and chromosomes occurs mostly when the cell is in the G2 phase. Recovery from sublethal damage occurs in all phases of the cell cycle. However, this is most pronounced in the S phase, which is also the most radioresistant phase [3].
15.3.2.4 Degree of Tissue Oxygenation
Molecular oxygen is known to have the ability to potentiate the response to radiation; this is known as the oxygen effect. The amount of molecular oxygen rather than the rate of oxygen utilization by the cells is the most important factor for increasing the sensitivity of cells to radiation. The probable mechanism is the allowance of additional free radicals, which enhance the damage of cells [5].
15.4 Radiation-Induced Cell Injury
In general, an injury which has a high chance of repair is sublethal, that which can be repaired with treatment is potentially lethal, and that which is permanent is lethal. The nucleus is relatively more radiosensitive than the cytoplasmic structures. Nuclear changes after radiation include swelling of the nuclear membrane and disruption of chromatin materials. Cytoplasmic changes include swelling, vacuolization, disintegration of mitochondria and endoplasmic reticulum, and reduction in the number of polysomes [2, 5]. Depending on the dose of radiation and the subcellular changes, along with the previously described factors, the potential effects on the cell vary (Table 15.2). After ionizing radiation exposure, cellular injury occurs in one of the following forms [6]:
Table 15.2




Types of cellular damage in relation to approximate radiation dose (Modified from [3] with permission)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree