Piero Picci, Marco Manfrini, Nicola Fabbri, Marco Gambarotti and Daniel Vanel (eds.)Atlas of Musculoskeletal Tumors and Tumorlike Lesions2014The Rizzoli Case Archive10.1007/978-3-319-01748-8_21
© Springer International Publishing Switzerland 2014
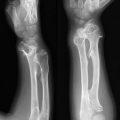
21. Biology of Giant Cell Tumor
(1)
Laboratory of Experimental Oncology, Istituto Ortopedico Rizzoli, Bologna, Italy
Abstract
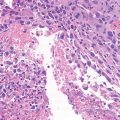
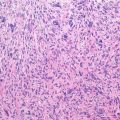
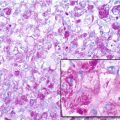
Giant cell tumor (GCT) of bone is a well-vascularized tissue made up of a network of spindle-shaped mononuclear stromal cells that represent the proliferating pattern, round mononuclear monocytes, and multinucleated giant cells.
Giant cell tumor (GCT) of bone is a well-vascularized tissue made up of a network of spindle-shaped mononuclear stromal cells that represent the proliferating pattern, round mononuclear monocytes, and multinucleated giant cells.
Mononuclear stromal cells are osteoblast-like cells, originating from bone marrow mesenchymal stem cells, positive to collagen 1, bone sialoproteins, osteonectin, osteocalcin, and alkaline phosphatase.
Giant cells are osteoclast-like cells originating from hematopoietic stem cells and expressing tartrate-resistant acid phosphatase (TRAP), cathepsin K, vitronectin receptor, calcitonin receptor, and CD68 and CD14 macrophage markers (Thomas and Skubitz 2009).
Besides tumor-derived factors (growth factors, BMP, etc.), mononuclear proliferating cells produce a component of tumor-necrosis factor (TNF) family, the membrane and soluble receptor activator for nuclear factor kB (RANKL), that interacts with RANK expressed on monocyte lineage and osteoclast precursors and, in presence of cytokines and macrophage colony-stimulating factor (M-CFS), induces osteoclast differentiation, thus shifting bone remodeling toward osteolysis (Wittrant et al. 2004).
In osteolytic tumors solubilization and digestion of inorganic and organic components by acid protease activity releases from bone matrix a very complex network of soluble factors (i.e. TGFb) and hormones that activate mononuclear cell proliferation creating a vicious circle where suitable targets for therapy can be identified (Broadhead et al. 2011). Previous results (Gamberi et al. 2004; Fujisaki et al. 2006) showed increased expression of urokinase-type plasminogen activation system, MMP9, and carbonate dehydratase 2 (CAII) activity in aggressive GCT when compared to latent forms (Enneking’s surgical system), and a recent study demonstrated that targeting osteoclast lineage by blocking RANKL binding to RANK, cell adhesion and migration, or enzymatic activities is a current therapeutic approach in bone tumor treatment. Interestingly, the authors also pointed out the additional direct role of RANKL in tumor cell proliferation through an independent osteoclast way (Heymann 2012).
Cytogenetically, the most common chromosome aberrations in GCTs are telomeric associations involving multiple chromosome ends that may be responsible for structural chromosomal aberrations, an important event in GCT recurrences. Genomic instability and aberrations almost exclusively in aggressive GCTs have been reported as well as translocations involving chromosomes 1, 2, 11p, 12, 18p, 13p, 15p, and 19 (Moskovszky et al. 2009; Gorunova 2009). Advances in molecular biology have allowed a better knowledge of the mechanisms involved in malignant progression and the detection of key molecules or pathways involved in tumor-host interaction, useful to identify a subset of high-risk GCT patients prone to receive a different clinical management in terms of a closer follow-up and medical adjuvant therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree