Fig. 6.1
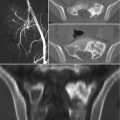
Initial imaging of sacral mass. (a) Sagittal radiograph demonstrating hypodense lesion immediately anterior to sacrum. (b) Computed tomography scan confirms a primary sacral mass with bony destruction

Fig. 6.2
Magnetic resonance imaging of sacral mass. Subsequent imaging modality revealed an encapsulated, heterogeneous mass emanating from the sacrum. (a) T1 phase and (b) T2 phase confirm heterogeneous density. (c) Axial view demonstrates clear plane between mass and gluteus maximus mm
6.2 Method of Biopsy
The need for biopsy is clear and absolute due to the multiple underlying histologies of neoplasm, both benign and malignant, within the sacrum. The method of biopsy, however, is less clear and the source of much controversy. Historical descriptions of biopsy technique have included open (incisional) biopsy, excisional biopsy, core needle biopsy, and fine needle aspiration with the last two performed by either image guidance or direct palpation. Recent experience has clarified the role of each with some significant pitfalls now recognized.
Incisional biopsy should only rarely be undertaken for the diagnosis of a sacral tumor. With modern image guidance and large bore core biopsy needles, there is no need for tumor disruption to obtain diagnosis. Incisional biopsy is fraught with complications, most severely the spread of tumor across planes of tissue not otherwise invaded by the malignant process. Local bleeding of the tumor and biopsy site allows for the diffuse spread of tumor cells and negatively impacts the outcome. Many times, a complete oncologic resection is not possible simply due to biopsy technique. This unfortunate outcome can be avoided with proper planning.
Small (<3 cm) tumors which have clear benign features on imaging can be biopsied by complete excision [1]. Using this method, adherence to oncologic principles is required in the event a high-grade neoplasm is identified on final pathologic analysis. Great care should be taken to not disrupt uninvolved tissue planes and ensure adequate hemostasis during dissection. The primary benefit of this technique is an adequate tissue sample for analysis by the pathologist. Architecture of the tumor is preserved maximally which allows for description of tumor heterogeneity and identification of poorly differentiated areas within the larger specimen.
Percutaneous biopsy can be performed by either fine needle aspiration (FNA) or core needle biopsy (Fig. 6.3). Prior to selection of technique, imaging should be reviewed directly between the treating physician and diagnostic radiologist focusing on two components. First, the a priori probability of metastatic disease should be determined. For those patients with a high probability of metastatic tumor, FNA is appropriate to confirm the suspicion. Second, the heterogeneity of the tumor should be discussed to ensure proper sampling of the biopsy. There should be a focus on any high-grade components, as these foci within the primary lesion can contain characteristic genetic alterations used to guide therapy.


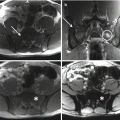
Fig. 6.3
Computed tomography-guided biopsy. A posterior approach was used to biopsy the lesion and confirm the histology as chordoma
Use of FNA for the diagnosis of soft tissue sarcoma is appropriate when performed in the setting of a multidisciplinary team. As mentioned above, the sampling of the tumor should be planned from the initial imaging and the biopsy should be performed under image guidance for tumors with significant heterogeneity. The pathologist should immediately assess adequacy of the specimen so that a core biopsy can be performed, if needed. Using this method, FNA is adequate for diagnosis in 77% of cases and 78% of the time the diagnosis correlates with malignant radiographic findings. For those lesions with benign features on imaging, FNA is not as helpful as the final diagnosis is in agreement in only 44% of cases. In the case of indeterminate image characteristics, FNA can differentiate benign from malignant lesions in 63% of cases [2]. FNA utility is increasing as subtypes are increasingly diagnosed by cytogenetic techniques since a relatively small number of cells is needed for analysis.
If resection of the tumor is considered in the treatment algorithm, then the surgeon should direct the biopsy plan after discussion with the diagnostic radiologist. Many times the biopsy tract can be placed within the planned incision, making excision at the time of tumor extirpation possible. At times, there are limitations of biopsy approach due to anatomic constraints and in those cases, placement of the biopsy tract outside the planned surgical incision cannot be avoided. It is not mandatory to excise a core biopsy tract if it will yield an unacceptable increase in morbidity but the biopsy cavity from an incisional biopsy should always be excised. Local recurrence (9%) following core biopsy without excision of the biopsy tract is rare compared to distant recurrence risk (25%) in the same patients [3].
6.3 Differential Diagnosis
6.3.1 Primary Benign Sacral Tumors
The primary consideration on biopsy of sacral tumors is whether a malignant process is responsible for the lesion identified on imaging. Not all lesions identified are malignant and several benign primary lesions occur within the sacrum. The most common benign tumor of the sacrum is a giant cell tumor (GCT), which is responsible for approximately 60% of benign lesions within the sacrum and 13% of all sacral tumors [4]. While only 7% of GCT involve the spine, the sacrum is the most common site of involvement within the spine [5]. The histologic hallmark of these lesions is the presence of large, osteoclast-like multinucleated cells (giant cells) scattered within spindle cell stroma. There are often hemorrhagic and fibrotic areas found within the tumor [6, 7]. Genetic hallmarks of GCT include telomere fusion, termed telomeric association (tas), which is found in 50% of GCT. Rearrangements of chromosome 17 (described below) have also been reported which hints at the presence of aneurysmal bone cysts within the larger lesion [7].
The second most common benign tumor found in the sacrum is the aneurysmal bone cyst (ABC). The spine is the location of approximately 20% of all ABCs and about 20% of spinal ABCs are located within the sacrum, which means the sacrum is the primary site for 3–4% of all ABCs [4–6, 8]. The histologic findings associated with ABC include large blood filled cystic spaces which are formed by septae of fibroblasts. There is little evidence of atypia within the cellular component of these lesions though mitoses can be common. The osteoclast-like giant cells seen in GCT are seen in ABCs as well but the large cystic areas distinguish these lesions from the former [7]. Cytogenetic rearrangements of the ubiquitin-specific peptidase 6 (USP6) gene on chromosome 17 are seen in 70% of primary ABC [7].
The spine is the primary location of osteoblastoma in 40% of cases and the sacrum is the site of 17% of these spinal lesions, thus the sacrum accounts for only 1% of all osteoblastomas [4, 5]. There are three classically described patterns of osteoblastoma on imaging and the most aggressive pattern of bone destruction with infiltration of adjacent structures carries a local recurrence rate approaching 50% [4]. This aggressive form is termed “epithelioid osteoblastoma” but there is no evidence that it has a worse prognosis compared to other subtypes [7]. Histologic hallmarks include disorganized spicules of woven bone lined with osteoblasts which do not demonstrate any degree of atypia [7]. There have been no genetic alterations described for osteoblastoma.
The final category of benign sacral tumors includes schwannomas and neurofibromas which arise from the neural elements of the sacrum. Schwannomas can be quite large at diagnosis with a mean diameter of 10.5 cm [4, 8]. Neurofibromas are often smaller tumors and can be distinguished by the lack of a capsule as opposed to the schwannomas which often are encapsulated. The malignant form of neurofibroma, called malignant peripheral nerve sheath tumor, can occur in patients with neurofibromatosis type 1. The hallmark schwannomas is the biphasic cellular components, termed Antoni A and B tissue. The former is characterized by spindle cells arranged in a compact group while the latter is more loosely arranged spindle cell foci within the tumor. This characteristic finding is readily identified and, when present with a fibrous capsule, is diagnostic for a schwannoma. The biphasic cellular component of the schwannoma contrasts the more loosely arranged spindle cell infiltrate of nerves found with neurofibroma. Genetic associations between these two entities include mutations in the NF2 gene which lead to schwannoma tumorigenesis and mutations in the NF1 gene which are found commonly within neurofibromas [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree