Fig. 1.1
Chiba aspiration biopsy needle. A thin-walled cannula with a beveled tip angled 25° is fitted with a matching stylet
The Hawkins (Cook) and TruGuide aspiration needles (Bard Biopsy System, Tempe, AZ) are equipped with a sharp trocar tip and a blunt-tipped stylet (Fig. 1.2) [29, 30]. These needles are particularly useful for getting past loops of small bowel and for reaching mesenteric nodes and masses. The blunt-tipped stylet may also prevent puncture of intervening vascular structures [30]. The sharp trocar stylet is used to get through the subcutaneous tissues and peritoneal wall. Once the cannula reaches the peritoneal fat, the blunt-tipped stylet is used to gently advance the needle. A gentle touch of the blunt stylet causes peristalsis, and this motion of the bowel clears the path to the target.


Fig. 1.2
The Hawkins and TruGuide aspiration needles are fitted with a sharp trocar tip (a) as well as a blunt-tipped (b) stylet. The stylet is shown on top of each figure, the cannula is shown in the middle, and the appearance of the stylet fitted in the cannula is depicted at the bottom of each figure
Cutting needles are used to obtain tissue samples for histologic analysis. These needles are further classified as end-cutting or side-cutting, depending on their design. The first-generation end-cutting needles were basically aspiration needles with a sharpened tip for cutting.
Several different needle types were developed with various beveled angles, needle tips, and stylet configurations [28]. The simplest end-cutting needle is a Menghini needle (Dyna Medical Corp., London, Ontario, Canada). It has a sharp, beveled convex tip with a 45° angle (Fig. 1.3). When moved forward into the tumor, it cuts out a cylinder of tissue [31]. The cutting tip of the Franseen needle (Cook) is serrated and has a sawtooth appearance (Fig. 1.4) [32]. With rotation and forward movement, the needle cuts into the tissue, collecting a small core biopsy sample. The Westcott needle (Cardinal Health, Dublin, OH) has a combined side-cutting and end-cutting mechanism. It has a side slot measuring approximately 2-mm long that is located a few millimeters from the tip of the needle (Fig. 1.5). The combination of the side slot and the cutting tip enables aspiration of a larger amount of tissue when compared to Menghini or Franseen needles [33].

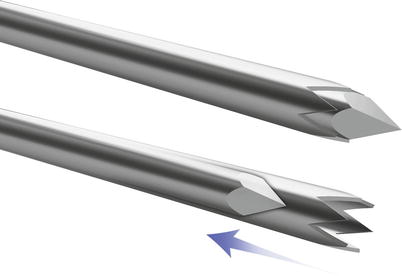
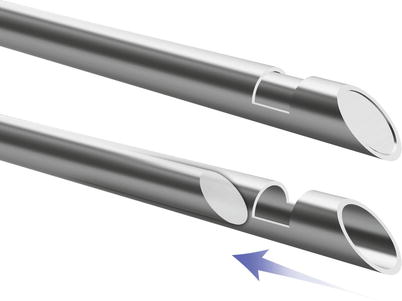

Fig. 1.3
Menghini-type needle has a sharp, beveled convex tip with a 45° angle. When moved forward into the tumor, it cuts out a cylinder of tissue

Fig. 1.4
Franseen-type needle has a serrated tip. With rotation and forward movement, the needle cuts into the tissue, collecting a relatively small core biopsy sample

Fig. 1.5
Westcott-type needle has a side slot near the tip of the needle. The dual sampling mechanism consisting of the side slot and the end hole allows for collection of larger amount of tissue compared to other end-cutting core biopsy needles
Cope and Abrams biopsy needles are examples of side-cutting needles used in pleural biopsy in patients with pleural effusion [34, 35]. Both of these needles have a notch near the tip. Once the needle assembly is introduced into the pleural cavity, the side-cutting edge of the needle is used to snag the pleura and collect a tissue sample. Multiple passes are often necessary to obtain an adequate sample. Cope and Abrams biopsy needles are often used in “blind” biopsy procedures without the use of imaging guidance. In the United States, blind biopsy procedures have primarily been replaced by image-guided biopsies using semiautomated cutting biopsy needles.
To increase the yield of cutting biopsy needles, a manual side-cutting needle was developed to cut a string of tissue [36]. The initial Tru-Cut needle consisted of an inner stylet fitted with a notch for trapping tissue and an outer beveled cannula for cutting the tissue. To obtain a biopsy sample, one would insert the whole needle into the edge of the targeted tissue, advance the inner stylet into the targeted tissue, and then move the outer cutting cannula manually over the stylet to cut the tissue trapped in the notch of the stylet. This would yield a semicylindrical piece of tissue.
In the more contemporary designs, the function of the Tru-Cut needle is automated in spring-loaded biopsy guns (Fig. 1.6) [37]. The automated devices obtain higher quality and more intact core biopsy samples than obtained with manual needles [37, 38]. Currently, side-cutting biopsy guns are available in a variety of sizes, ranging from 20 gauge (the smallest) to 14 gauge (the largest). The length of the tissue sample is determined by the length of the notch in the stylet, also referred to as the “throw” of the needle. Some automated biopsy devices have a set “throw” (e.g., 1 or 2 cm), whereas other devices have adjustable “throw” lengths (e.g., 1- and 2-cm options) (Fig. 1.7).
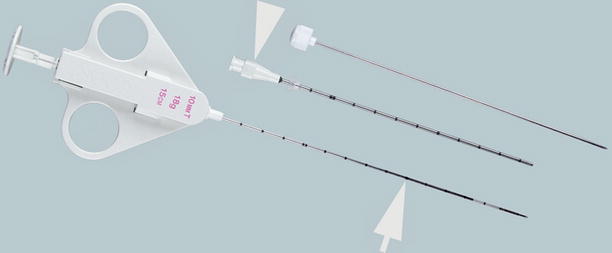
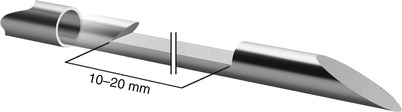

Fig. 1.6
Side-cutting core biopsy gun. QuickCore (Cook, Bloomington, IN) is a semiautomated, side-cutting core biopsy needle system (arrow). It may be used with a guide needle (arrowhead) during coaxial biopsy procedures (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)

Fig. 1.7
Semiautomated cutting core biopsy needles allow procurement of tissue cores of variable lengths. Some needles have a predetermined throw, whereas others have an adjustable throw
To operate a side-cutting biopsy gun, it has to be loaded prior to inserting it into the targeted tissue. For most devices, this is accomplished by pulling the stylet out until the spring is engaged. For needles with an adjustable throw (e.g., Temno; Cardinal Health, Dublin, OH), the trocar can be pulled until one click is heard for a short tissue sample or until two clicks are heard for a long tissue sample. The needle is then inserted into the edge of the tumor or just short of the targeted area within the organ of interest. In fully automated biopsy guns firing, the biopsy gun thrusts the inner stylet forward, followed almost instantaneously by the outer cannula. The specimen is cut and trapped in the side notch of the trocar. In semiautomated devices (e.g., QuickCore, Cook), the stylet is advanced gently into the tissue. Prior to firing the biopsy gun, additional imaging can be obtained to confirm that the needle is in the appropriate position. While holding the biopsy gun steady, the spring is released and the cutting cannula travels over the stylet, cutting a predetermined length of tissue that is trapped in the side notch of the trocar. The needle is then removed from the tissue. To harvest the specimen, the stylet is pulled back until the spring is engaged. The stylet is advanced gently to expose the sample for retrieval (Fig. 1.8). After retrieving the tissue sample, the needle is ready to be reinserted into the tissue to obtain additional samples.
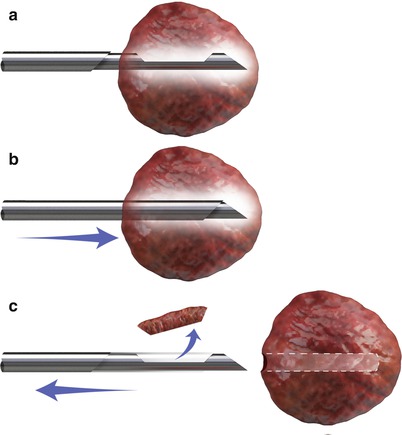

Fig. 1.8
Mechanism of a semiautomated cutting core biopsy needle. The stylet is advanced into the tumor (top). The position of the stylet can be confirmed by imaging. Once the biopsy gun is triggered, the cutting cannula travels over the stylet and traps the tissue in the groove of the stylet (middle). The biopsy sample is retrieved after removing the needle from the tissue (bottom)
The automated end-cutting biopsy needle (e.g., BioPince; Angiotech Pharmaceuticals, Vancouver, BC) has two cannulas. The tissue sample is obtained with a strong forward stroke of the cutting cannula. As opposed to the semicylindrical specimen obtained with side-cutting needles, end-cutting needles yield a cylindrical specimen that is slightly larger in diameter and volume for needles of similar gauge. One drawback of the end-cutting needle is a high rate of failure that results in no biopsy sample [39, 40]. This so-called zero biopsy may be more common with devices that have shorter throw lengths. Another disadvantage of automated end-cutting needles is that additional imaging cannot be obtained to confirm that the needle is in the appropriate position just prior to releasing the spring.
Needles larger than 18 gauge have been shown to increase the risk of pneumothorax during intrathoracic biopsy procedures [41]. In this case, a detachable 18-gauge cutting needle allows the largest possible sample to be collected without an increased risk of pneumothorax, potentially increasing the yield [42, 43]. Detachable cutting needles function as both a guide and a cutting needle. The notched stylet containing the specimen can be detached and removed while the cannula remains in place, allowing for multiple passes. However, reattaching the stylet to the cannula has been reported as cumbersome, and therefore these needles have not gained wide acceptance [42].
Transvenous Biopsy Devices
The rationale and technique for transvenous organ biopsy are further discussed in the next section. Briefly, liver biopsies in patients with irreversible coagulopathy or ascites are performed using the transvenous technique to avoid the risk of peritoneal bleeding. Initially, transjugular liver biopsies were performed using reverse-beveled end-cutting needles [44]. Contemporary transvenous biopsy devices use spring-loaded side-cutting needles (Fig. 1.9) [45]. A typical transvenous biopsy set (e.g., Cook) consists of the following essential elements: a multipurpose catheter for selective catheterization of the hepatic vein; a 7-French stiff cannula with an angled tip that can be pointed anterior or posterior; and a 60-cm long, side-cutting semiautomated biopsy needle with a 2-cm throw. Both 18- and 19-gauge needles are available. In addition, a 9-French angled-tip introducer sheath can help stabilize access in the hepatic vein during the procedure. A shorter version of the device is available for use in pediatric patients. A transvenous biopsy set is also available for renal biopsies in patients with irreversible coagulopathy, severe hypertension, or small atrophic kidneys [46]. A transjugular renal biopsy set (Cook) is comparable to the set used in transjugular liver biopsy with some modifications [47]. The stiff cannula is fitted with a decreased curvature to accommodate the renal vein, and the biopsy needle is 70-cm long and has a blunt tip to prevent perforation of the renal capsule (Fig. 1.10).
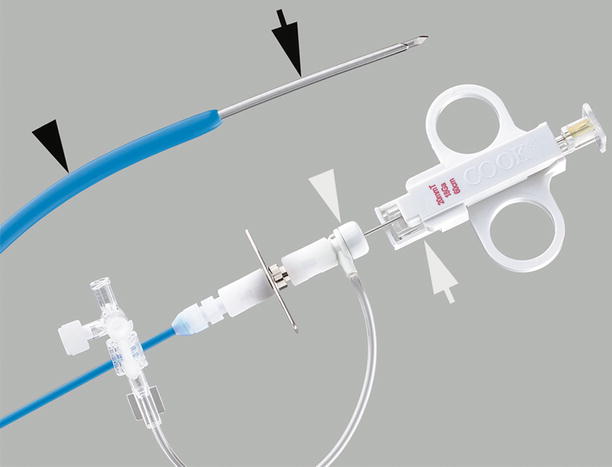
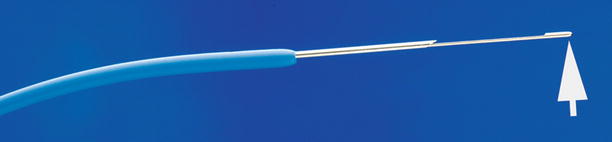

Fig. 1.9
Transjugular liver biopsy system. The 7-French stiff cannula is fitted with a hemostatic valve (white arrowhead) for introduction of the 18- or 19-gauge side-cutting core biopsy needle (white arrow). The distal end of the stiff cannula is gently curved (black arrowhead). When the handle is rotated, the tip of the stiff cannula faces the wall of the hepatic vein, which is then pierced by the needle tip (black arrow) (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)

Fig. 1.10
Transjugular renal biopsy system. In comparison to the transjugular liver biopsy set, the core biopsy needle has a blunt tip (arrow) to prevent perforation of the renal capsule (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)
When access to the hepatic or renal vein is not available using a superior approach, transvenous liver or renal biopsies can be performed from a femoral approach using biopsy forceps (Meditech) [48, 49]. The flexible forceps have oval-shaped, serrated cutting jaws. Biopsy samples are obtained by opening and wedging the jaws of the forceps into the targeted tissue (Fig. 1.11). A bite of tissue is trapped in the forceps when the jaws are closed. To cut the tissue, the catheter is pulled back into the introducer sheath. Once the biopsy forceps are removed from the patient, the jaws are opened to expose the tissue sample for harvesting.
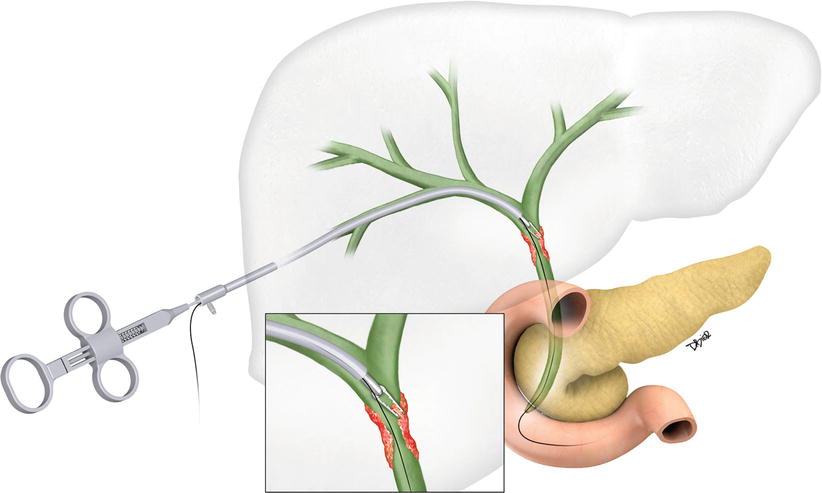

Fig. 1.11
Artist rendition of a forceps biopsy device used for sampling of a bile duct stricture. During the procedure, an introducer sheath is placed over a guidewire. The biopsy device is inserted alongside the guidewire. Once the forceps device is at the intended target, the sheath is withdrawn to expose the forceps. With the wings open, the device is advanced and wedged into the biopsy site. Once the forceps wings are closed, a bite of tissue is trapped. Pulling the device back procures the sample
Endoluminal Biopsy Devices
Biopsy of endoluminal lesions or strictures in the bile duct or ureter can be accomplished using biopsy forceps or brush biopsy devices [50, 51]. In the assessment of biliary or ureteral strictures, brush biopsy samples are more cellular than direct bile or urine samples owing to traumatization of the ductal epithelium or urothelium through contact with the abrasive bristles of the brush [52]. The brush biopsy device consists of a blunt-tipped flexible wire with a short, circumferential brush located near the tip of the wire (Fig. 1.12). The brush is contained within a 5-French housing catheter. An introducer sheath is necessary to maintain access in the bile duct or ureter while using the brush biopsy device.
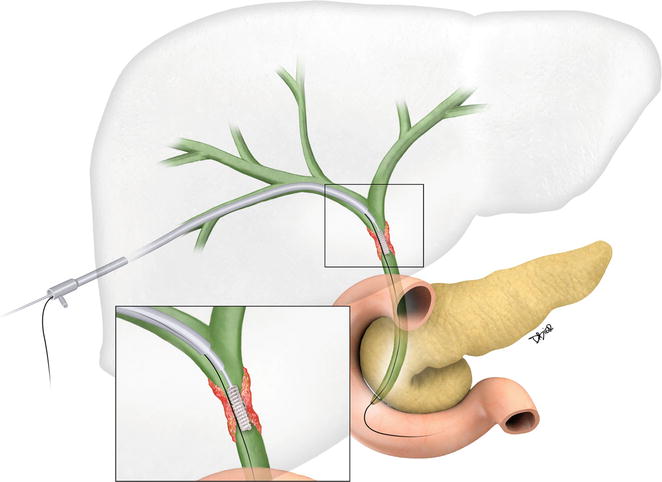

Fig. 1.12
Artist rendition of a brush biopsy device used for sampling of a bile duct stricture
Bone Biopsy Needles and Devices
Osteolytic lesions and tumors that destroy the bone can be biopsied using any of the soft tissue biopsy needles. Both FNA and cutting needles can obtain diagnostic samples. Special bone biopsy needles have been developed for sampling sclerotic bone lesions and for sampling targeted tissue that lies deep to an intact cortical surface. Stronger and possibly larger needles are needed under these circumstances. In general, most of these bone biopsy needles are designed to perform trephine biopsies of bony lesions with sufficient matrix [53]. An inadequate tissue sample volume or excessive fragmentation of the sample may lead to inconclusive pathology results. To sample a sclerotic lesion or to make an opening in the intact cortex of a bone, several needle designs have been evaluated.
The Jamshidi-type needles (Fig. 1.13) have a cannula and a trocar stylet that is fitted with a large sturdy handle to provide the operator with a good grip, allowing for application of pressure or turning of the needle as well as providing a surface where a light hammer can be used. Other bone biopsy needles have a serrated or saw-toothed cutting edge (Fig. 1.14), often used to sample hard sclerotic lesions. These needles are also fitted with a large handle to provide the operator with a good grip. The needle is advanced with the trocar stylet to the surface of the bone. Once the stylet is removed, the needle is turned while applying forward pressure to cut through the cortex or sclerotic bone. One drawback of this system is that to harvest the sample, the needle has to be removed completely from the bone.
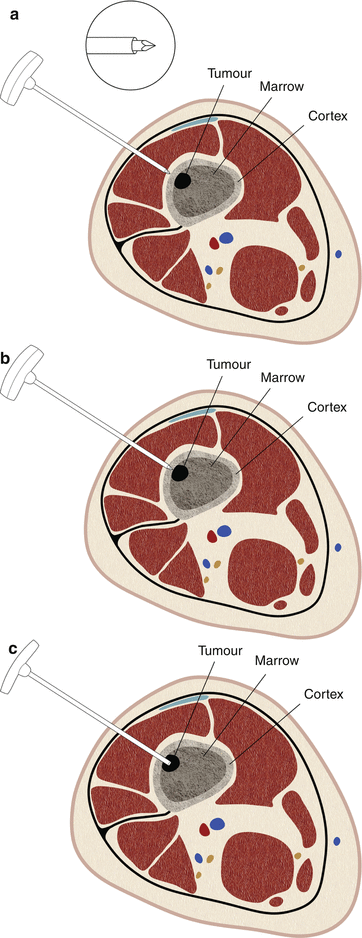
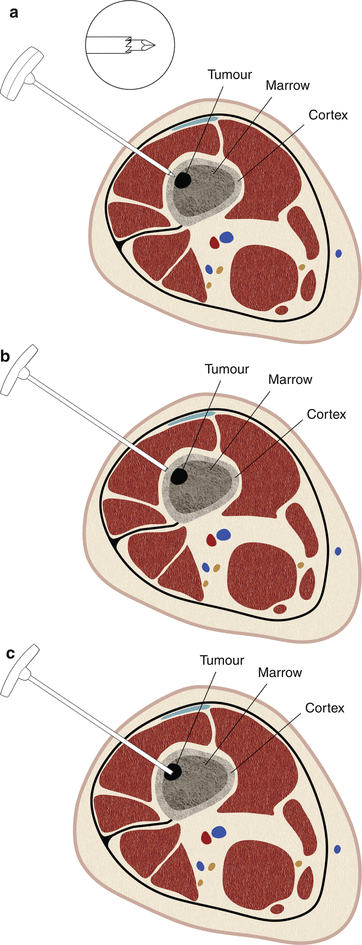

Fig. 1.13
Jamshidi-type needle for bone biopsy. These needles have a trocar stylet and a large sturdy handle (a). The needle is driven into the hard bone using a hammer (b), necessitating larger and stronger needle shafts. Usually, the smallest of these needles are 13 gauge in size. Once the cannula reaches the lesion deep to the cortex, the stylet is removed providing a conduit for placement of the sampling needle (c). If the tumor is lytic and has no bony matrix, any FNA or cutting core biopsy needle can be used for sampling. For sclerotic lesions, a Franseen-type needle with serrated tip can be used to obtain a trephine biopsy sample

Fig. 1.14
Bone biopsy needle for sclerotic lesions. The cannula in these needles is fitted with a saw-toothed cutting tip. A trocar stylet helps advance the needle through the soft tissues to the surface of the bone (a). The stylet is removed, and the needle is advanced by turning it as the serrated tip cuts into the cortex (b). As the needle is advanced into the sclerotic bone, the cannula is packed with hard bone (c). To harvest the sample, the needle has to be removed from the bone completely
The Elson bone biopsy needle (Cook) (Fig. 1.15) is designed for a modified coaxial technique (see below) where a 23-gauge needle with a removable hub is first inserted to the surface of the bone. Subsequently, a combination of a blunt-tipped cannula and a tapered obturator is advanced over the 23-gauge needle. A cutting needle with a serrated tip is advanced in coaxial fashion through the cannula. The cutting needle has a small disc-shaped handle, providing a grip that enables the operator to turn the needle. With this needle, and probably with other trephine biopsy needles, the cortical bone may clog the needle tip [54]. To obtain lesion tissue deep to the cortex, the needle should be unclogged by removing the impacted cortical bone. In this manner, additional biopsies from the lesion deep to the cortex will not lead to an impacted sample.
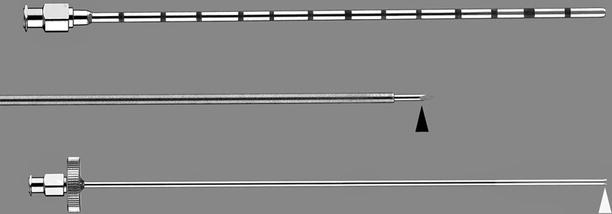

Fig. 1.15
Elson bone biopsy needle (Cook). This needle has three major components. First, a 23-gauge needle (middle) fitted with a trocar tip (black arrowhead) and a removable hub allows targeting of the tumor. Next, the cannula (top) fits over the 23-gauge needle as it is advanced to the edge of the bone. Finally, the biopsy needle (bottom) with serrated tip (white arrowhead) is advanced through the cannula to obtain a trephine biopsy sample. Multiple samples can be obtained through the cannula (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)
Other needles have a stylet that is shaped like an eccentric drill (Bonopty; Bone Biopsy System, Apriomed, Londonderry, NH) (Fig. 1.16). The stylet is introduced through the cannula in coaxial fashion and helps cut the cortex; the cannula is then advanced over the stylet to engage the bone (Fig. 1.17). In this fashion, the cannula acts as a guide needle for sampling the deeper tissue.

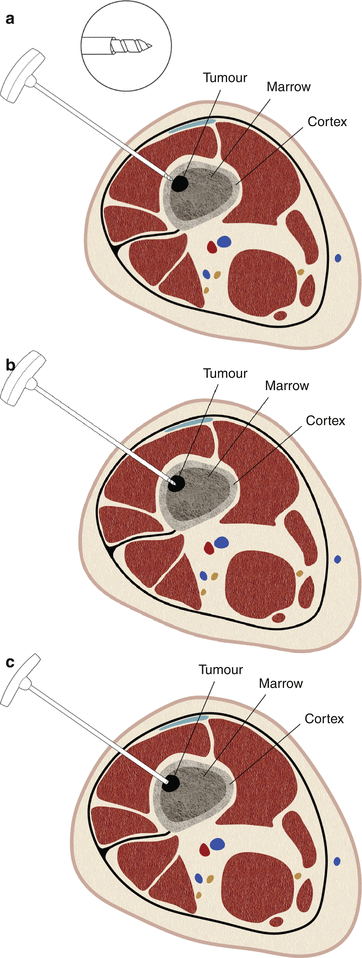

Fig. 1.16
Bonopty-type needles have a stylet that is shaped like an eccentric drill. The needle is driven into the bone by manually rotating it clockwise

Fig. 1.17
Bonopty-type bone biopsy needle. These needles have an additional trocar stylet (not shown) to help advance the needle through the soft tissues. Once the cannula is positioned against the bone, the trocar is replaced with the drill stylet (a). As the whole needle is turned clockwise, the drill bit engages the bone (b). The stylet is removed, providing a conduit for coaxial biopsy needles and sampling of the lesion (c). The cannula must be removed from the bone carefully to avoid fracturing the tip
And finally, some needles have a threaded cutting cannula (Ostycut, Bard) (Fig. 1.18). A trocar stylet helps guide the needle to the surface of the bone. Once the stylet is engaged in the sclerotic bone, the cannula is turned to engage the bone. Turning the cannula further advances the needle into the hard bone.
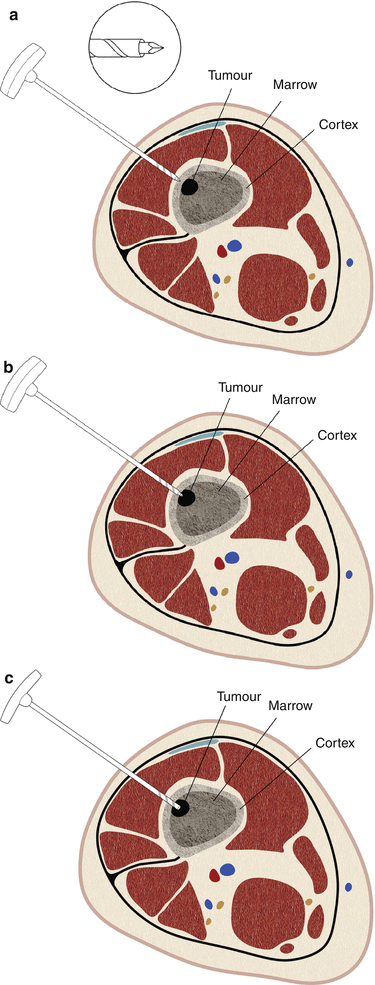

Fig. 1.18
Bone biopsy needle with a threaded cannula. These needles have a trocar stylet. The cannula is threaded to engage the cortex (a) and to drive the needle into the bone (b). Once the tip is deep to the cortex, the stylet is removed (c), providing a conduit for biopsy needles
For lesions that lie deep to an intact cortex and for sclerotic bone lesions, a handheld drill may be used to penetrate the bone [55]. Handheld drills are either manual or electrical, usually battery operated. The OnControl Bone Marrow Biopsy System (Vidacare Corporation, Shavano Park, TX) consists of a driver and biopsy needle set (Fig. 1.19) [56]. The battery-powered driver resembles a small handheld drill, weighs 315 g, and fits into a sterile bag fitted with an adaptor. Bone biopsy needles are 11 or 15 gauge and consist of a diamond-tipped stylet and a biopsy cannula with serrated tip. The hub of the needle is designed to fit into the adaptor. The device helps advance the needle with ease into the sclerotic bone.


Fig. 1.19
Handheld drill for bone biopsy. The reusable, battery-powered driver of the OnControl Bone Marrow Biopsy System (Vidacare Corporation, Shavano Park, TX) (a) is placed in a sterile plastic cover (not shown) for each procedure. The hub of the disposable biopsy needle (b) fits into the drill (Published with permission from Vidacare Corporation)
Treated Needles for Use Under Ultrasound Guidance
Visibility of the needle on ultrasonography may be limited in tissues with increased echogenicity, deeper tissues, or when access is limited by overlying air or bone [57]. A poor angle of sonication (<20°) and a small-gauged needle also contribute to suboptimal visualization of the needle on ultrasonography [57]. Under these circumstances, treated needles facilitate detection and monitoring of the needle tip as it is advanced through the overlying tissues to the targeted tissue. There are several strategies to improve needle visibility under ultrasound visualization. Teflon (Cook) or echogenic polymer coating (Echo-Coat, STS Biopolymers, Inc., Henrietta, NY) on the biopsy needle helps visualize the entire shaft of the needle. One drawback of this technology is that over time the needles lose their coating, presumably owing to resorption of microbubbles by the surrounding fluid during the biopsy. Manufacturers have used dimpled (Fig. 1.20), etched-tipped (Cook), or screw-tipped stylets (Inrad, Grand Rapids, MI) to improve needle visibility ultrasonography. Visibility is usually limited to the last few millimeters of the needle’s length. In general, coated needles seem to provide better echogenicity and better visualization than do partially etched or dimpled needles, particularly at small angles of insonication [57]. The diagnostic yield of ultrasound-guided biopsies is similar for treated and untreated needles; but with better visualization of the needle tip, the procedure may take less time and be safer for patients [58].
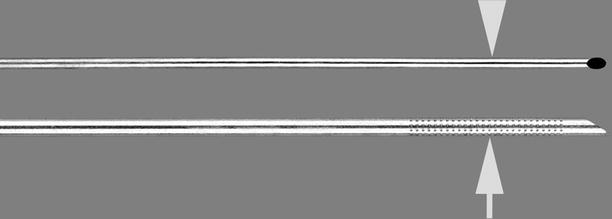

Fig. 1.20
Chiba aspiration biopsy needle with echogenic tip. The distal end of the cannula (arrow) is dimpled to improve its visibility under ultrasonography. The Chiba needle has a stylet that matches the 25° angle of the cannula (arrowhead) (Permission for use granted by Cook Medical Incorporated, Bloomington, Indiana)
MRI-Compatible Needles and Devices
Most biopsy needles are made of stainless steel and are not MRI compatible, as the needles may become projectiles in the presence of a strong magnetic field. Other alloys may be relatively MRI safe but may still create a ferromagnetic susceptibility artifact that is too large and is unacceptable for imaging purposes. As experience with MRI-guided biopsies grows and MRI-guided interventions gain acceptance in the interventional radiology community, more MRI-compatible needles and devices are being introduced on the commercial market. Currently, MRI-compatible needles are usually made of titanium alloys. These needles create minor artifacts, estimated roughly at twice the size of the needle [59]. But the exact thickness of the needle artifact depends on the magnetic field strength and specific imaging parameters [60]. Titanium needles are softer and more pliable, do not track as well as stainless steel needles, and may not be as sharp as their stainless steel counterparts [61]. Both aspiration and semiautomated side-cutting titanium needles are available for MRI-guided biopsies (Invivo, Gainesville, FL). A trephine bone biopsy set is available with a serrated-tipped cannula, allowing biopsy of sclerotic bone lesions (Invivo). An MRI-compatible handheld drill is commercially available to create an opening in the cortical bone and to sample sclerotic bone lesions (Invivo). The drill is operated using a piezoelectric motor and is fully MRI compatible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree