Karen T. Brown
Because the hepatic artery provides most blood flow to hepatocellular carcinoma (HCC), with the portal vein providing trophic blood supply to the liver parenchyma,1 and the paucity of other effective nonsurgical treatments, it is not surprising that transarterial methods of treating unresectable HCC have become a mainstay of therapy over the last four decades. One of the early reports of transcatheter management of HCC by Charnsangavej et al.2 in 1983 described results following two methods of treatment. The first group received continuous hepatic arterial infusion (HAI) of floxuridine, doxorubicin, and mitomycin C in 14 patients requiring a 5-day hospital stay, with two courses of treatment given at 4- to 6-week intervals. The second group of 9 patients (2 of whom crossed over from the HAI group) was treated with hepatic artery embolization (HAE) using Ivalon (Unipoint Industries, High Point, North Carolina). Response rates in the two groups were similar: 71% in HAI group versus 67% in HAE group, with median survival of 12.3 months and 17.4 months, respectively. Later that year, Yamada et al.3 described the use of gelatin sponge cut into 1- to 2-mm pieces and “permeated” with mitomycin C or doxorubicin to treat 120 patients with HCC, infusing this chemotherapy-soaked gelatin sponge into the artery feeding the tumor. The 1-, 2-, and 3-year cumulative survival rates were 44%, 29%, and 15%, respectively. Yamada et al.3 noted that given the knowledge that HCC derives blood supply from “only the hepatic artery,”1 embolization can be expected to result in selective necrosis of the tumor tissue. They also suggested that although the effect of the single dose of mitomycin or doxorubicin may have been enhanced, it is possible that ischemia might be the primary mechanism of tumoricidal effect.3 In a 1987 study by Takayasu et al.,4 patients with HCC were divided into three groups for intra-arterial treatment. Group A received intra-arterial Lipiodol (Guerbet USA, Bloomington, Indiana) alone; group B, an emulsion of Lipiodol with doxorubicin; and group C, the same emulsion followed by embolization with gelatin sponge. Group C showed the best therapeutic effect; there was no significant difference in response between groups A and B, with practically no therapeutic effect from Lipiodol alone. Although these results can be interpreted in several ways, the authors pointed out that the results achieved using the anticancer emulsion plus gelatin sponge were superior to those previously described for embolization with gelatin sponge and anticancer agents and posited that the combination of Lipiodol blocking small vessels and gelatin sponge occluding more proximal feeding arteries resulted in an anoxic state “independent of drug sensitivity of the tumor.” They also noted an association between uptake of Lipiodol by the tumor and therapeutic effect. In 1989, Nakamura described a similar effect on survival, comparing 100 patients treated with doxorubicin, iodized oil, and gelatin sponge to 104 historical controls embolized with gelatin sponge and either doxorubicin (96) or mitomycin (8) achieving 1-, 2-, and 3-year survivals of 53%, 33%, and 18% in the iodized oil group and 45%, 16%, and 4% in those treated with only gelatin sponge plus chemotherapy. Taken together, these findings confirm that transcatheter treatment of HCC brought about a radiologic response; however, several questions remained unanswered. What was the primary driver of that therapeutic effect: chemotherapy or ischemia? Does Lipiodol indeed prolong the intratumoral dwell time of chemotherapy? And, finally, does an imaging response to treatment translate into improved survival?
Pharmacokinetic data supporting the use of intra-arterial chemotherapy or chemotherapy plus Lipiodol administered as an emulsion with an embolic agent, as commonly used today, is less than convincing. Studies clearly demonstrating high and prolonged concentration of chemotherapeutic agents within the tumor were performed using mitomycin C, doxorubicin, and aclarubicin dissolved in hydrocarbon solvents and then in Lipiodol5 or using a lipophilic agent,6 methods which are not used clinically. In the animal study by Konno,5 when the chemotherapeutic agent was dissolved in water and then mixed with Lipiodol and administered as an emulsion, concentration of drug in the tumor was high immediately but low at 6 hours, 1 day, and 7 days. In a study of 18 patients by Raoul et al.,7 doxorubicin was given to patients intra-arterially using three different methods: alone as an infusion, emulsified with Lipiodol, or with Lipiodol and gelatin sponge. There was no significant difference in total amount of doxorubicin released into the circulating blood, but patients in whom gelatin sponge was used had less released within the first hour of treatment. Another study evaluated intra-arterial doxorubicin versus doxorubicin with Lipiodol8 and found no difference in the area under the concentration-time curve, or terminal half-life, and no difference in pharmacokinetic profile or systemic toxicity using the same dose schedule compared to administering the doxorubicin intravenously. Even if the pharmacokinetic profile was more encouraging, none of these anticancer agents had ever been demonstrated to have a significant effect on the survival of patients with HCC when administered intravenously, making it difficult to know which agent, or which combination of agents, to use clinically. There are many embolic agents available for embolization. This has led to myriad methods of transcatheter intra-arterial therapy for HCC, limiting the ability to compare results between different groups. Meta-analysis by Simonetti et al.9 in 1997 failed to support the effectiveness of nonsurgical treatments for HCC, but some evidence of “moderate benefit” emerged from trial using tamoxifen and transcatheter arterial embolization (without iodized oil). Combining the heterogeneous population of patients with HCC who present with varied etiology and stage of underlying liver disease, size and number of tumors, and vascular invasion status, with the diverse methods used to treat them, made it even more difficult to compare results of treatment between diverse investigators, and there were no strong randomized trials.
The lingering question of whether reported radiologic response would translate into survival benefit was addressed with two randomized trials reported in 2002, which compared transarterial therapies to supportive care. Lo et al.10 treated a group of 80 patients, most had underlying hepatitis B, with either transarterial chemoembolization (TACE) using an emulsion of cisplatin and Lipiodol (Lipiodol Ultra-Fluide; Guerbet, Bloomington, Indiana) with gelatin sponge particles or best supportive care, using survival as the primary end point. There were 40 patients in each group; the TACE group received a total of 192 courses of embolization, median 4.5 per patient. TACE was associated with a significantly better actuarial survival of 57%, 31%, and 26% at 1, 2, and 3 years compared to 32%, 11%, and 3% in the best supportive care group. Later that same month, Llovet et al.11 published a randomized trial with three arms: chemoembolization with doxorubicin, lipiodol, and gelatin sponge; gelatin sponge alone; and best supportive care. Again, the primary end point was survival; response was a secondary end point. The study design was sequential; patients were assessed every 3 months and the trial was stopped when a significant survival advantage was demonstrated for the chemoembolization group. At the time the study was stopped, the Z value of the sequential triangular test for the embolization group remained within the triangular boundaries, indicating the need to recruit additional patients to achieve a valid conclusion. Survival probabilities at 1 and 2 years were 75% and 50% for embolization, 82% and 63% for chemoembolization, and 63% and 27% for symptomatic treatment, respectively. Although the authors believe that chemoembolization leads to a survival benefit compared to embolization alone, this could not be concluded statistically because the study was stopped before such a benefit could be demonstrated or refuted. Additional patients would have needed to be recruited into the embolization arm to reach a valid conclusion with regard to the impact of embolization versus symptomatic treatment. Of interest, 30 patients treated achieved an objective response by imaging: 16 were in the embolization group, despite the fact that only 35 patients in that group were treated, and the other 14 were among the 40 patients treated with chemoembolization. Although this study provides level 1 statistical evidence that chemoembolization offers a survival benefit compared to best supportive care, it does not allow for any statement regarding the effectiveness of chemoembolization versus embolization alone or embolization versus best supportive care.
That same year, Camma et al.12 published a meta-analysis of randomized chemoembolization trials. There were 18 randomized controlled trials pooled for analysis. The authors concluded that “chemoembolization significantly reduced the overall 2-year mortality rate (odds ratio, 0.54; 95% CI: 0.33, 0.89; P = .015) compared with nonactive treatment . . . overall mortality was significantly lower in patients treated with transarterial embolization (TAE) than in those treated with transarterial chemotherapy (odds ratio, 0.72; 95% CI: 0.53, 0.98, P = .39) and that there is no evidence that transarterial chemoembolization is more effective than TAE (odds ratio 1.007; 95% CI: 0.79, 1.27; P = .95), which suggests that the addition of an anticancer drug did not improve the therapeutic benefit.”12
If indeed the primary effect of transarterial treatment is from ischemia rather than a local chemotherapeutic effect, it follows that methods that maximize ischemia should be adopted. To that end, calibrated microspheres that are capable of occluding intratumoral vessels should be employed for HAE. In a very elegant study using iron oxide labelled Embosphere Microspheres (Merit Medical Systems, Inc., South Jordan, Utah) published in 2008, Lee and colleagues13 looked at the distribution of 100- to 300-μm and 300- to 500-μm spheres after embolization of VX2 tumor in rabbits. They found that on both magnetic resonance and histology, only the smaller 100- to 300-μm particles penetrated the tumor, whereas the 300- to 500-μm particles were found outside the tumor. Presuming that occlusion of intratumoral vessels will result in the most pronounced ischemia and resultant coagulation necrosis, these finding suggests that 100- to 300-μm particles or smaller should be used for embolization of liver tumors. Indeed, in 2008, Maluccio et al.14 described results obtained in 322 patients thus treated at Memorial Sloan Kettering Cancer Center using small particle embolization alone. Median survival was 21 months, with 1, 2, and 3 years overall survival of 66%, 46%, and 33%, respectively. When patients with extrahepatic disease or portal vein tumor were excluded, the overall survival in the 159 patients with liver-only disease rose to 84%, 66%, and 51% at 1, 2, and 3 years, respectively, with a median survival of 40 months. These results are surprisingly (or perhaps not so surprisingly) similar to the 1- and 2-year overall survival with chemoembolization described by Llovet et al.11 (82% and 63%) and are some of the best results ever reported for transcatheter treatment of HCC.
DEVICE/MATERIAL DESCRIPTION
Small calibrated microspheres without chemotherapy or Lipiodol are used to embolize HCC and other hypervascular tumors. Embosphere Microspheres are preferred by the author because of a demonstrated low incidence of postembolization occlusion of hepatic vessels.15 These particles are made from trisacryl cross-linked acrylic copolymer combined with gelatin and are hydrophilic and compressible. They received 510(k) clearance in the United States in 2000 and are U.S. Food and Drug Administration (FDA) approved for uterine fibroid embolization (UFE). Having been available for almost 15 years, these microspheres are some of the most studied embolic agents currently in use and are available in sizes ranging from 40 to 120 μm to 900 to 1,200 μm. There are other calibrated spherical embolics that can be used for HAE including Bead Block (Biocompatibles UK LTD), available in sizes ranging from 100 to 300 μm to 900 to 1,200 μm and produced from a biocompatible polyvinyl alcohol (PVA) hydrogel, and Embozene Microspheres (CeloNova BioSciences, Inc., San Antonio, Texas), available in sizes ranging from 40 to 900 μm and composed of a hydrogel core with a surface modification of Polyzene-F, a biocompatible polymer. Bead Block received 510(k) approval for neurovascular embolization in 2004 and is now cleared for use in hypervascular tumors. Embozene Microspheres are the most tightly calibrated microspheres currently available. Embozene Microspheres received 510(k) approval from the FDA in December 2008 for use in hypervascularized tumors.
TECHNIQUE
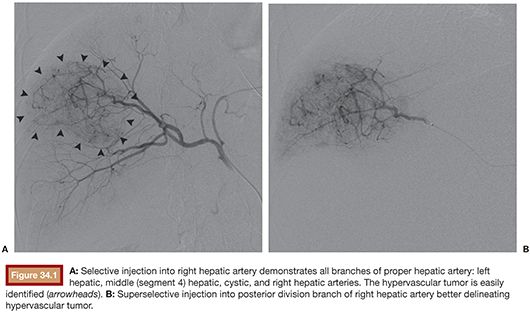
After performing celiac and superior mesenteric angiography to define the direction of portal blood flow, arterial anatomy, and primary blood supply to the tumor(s), a catheter is placed selectively into the vessel or vessels supplying the tumor to be treated. Embolization is performed as selectively as possible often using a coaxial catheter. The catheter is advanced into the vessel(s) to be treated (Fig. 34.1), and the microspheres suspended in contrast and maintained in suspension by admixing through a three-way stopcock (Fig. 34.2) are injected under constant fluoroscopic control until stasis is evident.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








