Key Facts
- •
Musculoskeletal complications may occur following otherwise successful renal, cardiac, or lung transplantation and may result in a diminished quality of life.
- •
Hyperparathyroidism may persist after renal transplantation and parathyroidectomy becomes necessary in a small percentage of cases.
- •
Osteoporosis is a frequent complication in post-transplant patients. It is estimated that over 40% of renal transplant recipients followed more than 5 years suffer an osteoporotic fracture.
- •
Gout occurs in up to 7.6% of renal transplant patients.
- •
Post-transplant bone-marrow edema syndrome is a cause of severe bilateral distal lower extremity pain in transplant recipients. This syndrome usually occurs early in the clinical course. Its cause is uncertain. Bone marrow edema and, in some cases, insufficiency fractures, are demonstrated on magnetic resonance imaging.
- •
Pretransplantation bone loss is an important predictor of post-transplantation fracture risk. To prevent the development of post-transplantation osteoporosis, attention should be focused on optimizing bone metabolism and bone mass prior to transplantation.
- •
Osteonecrosis has become less frequent after transplantation owing to changes in immunotherapy.
The modern era of organ transplantation began in 1954 with the successful transplantation of a kidney from one brother to his twin at the Brigham and Women’s Hospital in Boston, Massachusetts. Now, more than 25,000 solid organ transplants are performed yearly in the United States and the types of organs and tissues transplanted are increasing. The advances in transplantation have led to a corresponding need for imaging of these patients after surgery. This chapter reviews the major musculoskeletal complications that may occur following renal, lung, or cardiac transplantation. Selected treatment options are mentioned, but the reader is referred to other sources for detailed treatment plans.
Organ transplantation is the optimal form of therapy for those with many forms of irreversible chronic diseases such as chronic renal disease, hepatic failure, cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and congestive heart failure. Advances in immunosuppressive medications have improved allograft function over the last several decades and have significantly increased survival. The cost of containing once life-threatening complications such as rejection has been the increase in side effects, including metabolic bone disease, resulting from immunosuppressive medications.
Post-transplantation bone disease is a generic term referring to the spectrum of transplantation-related disorders of bone metabolism and function. The most important of these skeletal complications are the development of osteoporosis and related fractures, osteonecrosis (avascular necrosis), and diffuse bone pain. The importance of bone-related complications after organ transplantation was directly assessed by Navasa et al. His results underline the clinical and socioeconomic relevance of transplantation-related bone disorders, proving that these have a detrimental effect on the quality of life of patients by causing pain, imposing limitations in activities of daily living, and producing emotional stress. These individual effects also create a significant burden on the public health system due to the direct and indirect costs of treatment.
Bone disorders related to organ transplantation have similar pathophysiologic mechanisms, leading to common endpoints such as osteoporosis ( Box 41-1 ). However, each organ has unique epidemiologic characteristics and specific risk factors that can be highlighted.
- •
Older age
- •
Calcium deficiency
- •
Vitamin D deficiency
- •
Inactivity
- •
Excessive alcohol/tobacco
- •
Medicines (loop diuretics, anticoagulants, glucocorticoids)
- •
Renal or liver failure
- •
Immunosuppression
In liver diseases, the development of fractures has been a long-recognized clinical complication. Interestingly, the prevalence of fracture after liver transplantation is markedly higher among patients with cholestatic diseases (6%), such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC), than it is after hepatitis or other liver diseases (1%).
Patients with chronic heart failure have been found to have increased risk of low bone mass attributed to multiple factors including prolonged immobility, anorexia, hypogonadism, the use of loop diuretics, and vitamin D deficiency. Additionally, a trend has been noted for a higher rate of incident vertebral fractures among patients with ischemic cardiomyopathy, in comparison to those with dilated cardiomyopathy or other cardiac diseases, suggesting that vascular changes due to atherosclerosis may also be related to bone metabolic alterations and increased risk of osteoporosis.
Patients with chronic lung disease have a significantly increased risk of low bone mass and osteoporosis before transplantation. These are secondary to pre transplant exposure to glucocorticoids and a high prevalence of vitamin D deficiency, which has been found in up to 36% of patients with CF, and in 20% of patients with other pulmonary diseases (see Box 41-1 ).
Patients with severe chronic kidney disease who undergo renal transplantation now have 1-year survival exceeding 90%. Improving the long-term quality of life for renal transplant patients, including the prevention and treatment of bone-related disorders has become a major goal in the post-transplantation period. The risk factors for developing transplant-related bone disease are multiple in nephropathic patients. The most important is renal osteodystrophy, followed by secondary hyperparathyroidism, osteomalacia, adynamic bone disease, aluminum bone disease or β 2 -microglobulin amyloidosis, all contributing to distortion of osseous architecture and increased risk for fractures and bone pain ( Figure 41-1 ).


MECHANISMS
In organ transplantation, the primary goal is to prevent or inhibit donor-organ rejection by the recipient’s immune system. This has been achieved through the administration of immunosuppressants, of which the most used and best understood are glucocorticoids.
Unfortunately, there is strong indirect evidence that glucocorticoid therapy is one of the major etiologic factors for the development of severe post-transplantation osteoporosis. The first 6 months after transplantation is the period with the highest rate of bone loss and the highest incidence of fractures; it is also the time interval in which the highest doses of glucocorticoids are administered. Several studies on dynamic changes of bone mass after liver transplantation support the observation that bone loss after transplantation predominantly occurs during the first 6 months, with a more variable course afterward, resulting in recovery of some bone mass. Porayko et al. reported that fractures occurred predominantly within a short time period after transplantation: 57% of the fractures occurred within the first 6 months, 24% between 6 and 12 months, and only 19% developed more than 12 months after transplantation.
Bone loss after transplantation occurs predominantly during the first 6 months when corticosteroid doses are highest.
It has been suggested that initially, increased bone resorption after transplantation may be related to the effect that glucocorticoids exert on the synthesis of osteoprotegerin (OPG), decreasing its expression by osteoblasts leading to increased bone resorption. Osteoprotegerin is a decoy receptor for the RANK ligand (RANKL) and is produced by several cell types including osteoblasts and arterial cells. OPG blocks the RANK-RANKL signaling pathway, inhibiting osteoclastogenesis and osteoclast function resulting in a decrease in bone turnover. Hofbauer et al. assessed the effects of glucocorticoids on OPG and RANKL expression in human osteoblastic lineage cells, and found that dexamethasone inhibited OPG and stimulated RANKL production by osteoblastic lineage cells, indicating a potential mechanism for glucocorticoid-induced bone loss with further evidence suggesting that OPG has a role in the development of post-transplantation osteoporosis.
In early phases, when corticosteroid doses are high, there is suppression of osteoblastic-mediated bone formation and an increase in osteoclastic bone resorption. Later, when corticosteroid doses are low, there is an increase in bone formation with recoupling of bone remodeling.
Another category of widely used immunosuppressants are the calcineurin inhibitors cyclosporine (CsA) and tacrolimus. These drugs have a significant impact on preventing organ rejection and preserving life. One of the drawbacks to their use, however, is their propensity to cause rapid and profound bone loss. CsA and tacrolimus are inhibitors of calcineurin. They require binding to intracellular proteins—CsA to cyclophilins and tacrolimus to FK binding proteins. The resulting complexes inhibit the intracellular phosphatase calcineurin, which prevents transcription of T-lymphocyte cytokine genes and genes that control membrane molecules such as CD40 ligand. Calcineurin is a serine-threonine phosphatase that is uniquely regulated by calcium and calmodulin. Calcineurin interacts with nuclear factor of activated T cells (NF-AT), which constitute a family of transcription factors necessary for activation of genes involved in the inflammatory and immune system. Thus, inhibiting calcineurin, CsA and tacrolimus prevents activation of NF-AT with consequent inhibition of growth and differentiation of T lymphocytes critical to the immune response.
It has become evident that T cells are implicated in bone remodeling and that they appear to be a prerequisite for the development of CsA-induced osteopenia. T-lymphocyte numbers and subsets are altered, and decreased T-lymphocyte populations have been associated with increased osteoclast formation, producing increased bone turnover with predominant resorption over formation resulting in a decreased bone mineral density (BMD). Calcineurin promotes T-cell growth and differentiation, which induces osteoblast activation.
Guo et al. proposed a model in which bone loss after transplantation results from an increase in the rate of bone turnover in association with remodeling imbalance. This imbalance is in part a consequence of the inhibition of bone formation by glucocorticoids, and it is likely that this is a result of an increase in the rate of osteoblast apoptosis. This model applies to the early post-transplantation period, since the later phase of transplantation-related bone loss does not include the effects of glucocorticoids.
Doses of corticosteroids are usually sharply reduced after the first 6 months and may be stopped 1 to 2 years after surgery.
Proposed mechanisms to explain elevated bone turnover late after transplantation involve a decrease in glomerular filtration rate and subsequent elevation in creatinine resulting in an increase in osteocalcin, a persistently elevated PTH, as well as changes in serum calcium and decrease in calcitriol.
The increase in bone turnover after transplantation may result from a number of factors, including hypogonadism, immunosuppressants such as cyclosporine, and increased parathyroid hormone (PTH) secretion. Hypogonadism is most marked in men during the first few months after transplantation. This time course would be consistent with an effect of glucocorticoids, which suppress testosterone by a direct effect on the testis and by inhibiting the secretion of gonadotrophins. There is also a decrease in the production of adrenal androgens, as shown by a decrease in dehydroepiandrosterone sulfate (DHEA-S), resulting from adrenal suppression by glucocorticoids. Testosterone has direct effects on bone formation and resorption, but more importantly, it acts as a substrate for the production of estrogen (by aromatase) and hence inhibits bone resorption. Thus, low levels of testosterone (and DHEA-S) might be expected to increase bone turnover and cause a relative reduction in bone formation. The increase in PTH is multifactorial. PTH increase may be secondary to decreased renal function prior to transplant. PTH increase may be related in part to the inhibition of intestinal calcium absorption secondary to high-dose glucocorticoids and a decline in synthesis of calcitriol that is associated with renal impairment. Although excellent immunosuppressive drugs, cyclosporine, and tacrolimus are well known to cause renal toxicity in a dose-dependent fashion.
SKELETAL PRETRANSPLANT ASSESSMENT AND TREATMENT
Optimization of skeletal health should be a priority in patients awaiting a solid organ transplant. The assessment at or around the time of transplantation should include a complete clinical evaluation with focus on any history for remote or current fractures, oligomenorrhea, and a nutritional evaluation, with estimation of calcium intake.
Laboratory tests should include a comprehensive chemistry panel with serum phosphate, liver function tests, thyroid function, 25(OH) vitamin D, testosterone in males, and estrogen in females. In patients with renal failure, there should also be measurement of parathyroid hormone, which can be used as a surrogate for bone turnover. Low parathyroid hormone may predict low bone turnover and bisphosphonates have more potential to do harm in this subgroup. Urinary 24-hour calcium levels to assess for hypercalciuria could be helpful. Hypercalciuria predisposes to osteoporosis, and could be improved with thiazides which reduce urinary calcium losses.
A skeletal imaging file comprising anteroposterior and lateral radiographs of the thoracic and lumbar spine and DXA BMD measurements of the lumbar spine, hip, and the radius are suggested. The addition of the forearm measurement provides assessment of cortical bone density that may be affected by bone loss in hyperparathyroidism. The other sites allow assessment of trabecular bone that are especially sensitive to bone loss leading to osteoporosis.
A nutrition plan should be tailored to provide adequate calories and protein with avoidance of excess magnesium. The adjustment of calcium supplements should consider the urine calcium and PTH. The active metabolites of vitamin D are reserved for patients with malabsorption and secondary hyperparathyroidism; otherwise, 400 to 1000 IU per day of cholecalciferol are commonly administered doses.
Implementing an exercise program might be challenging or not even feasible, but this should be a goal in the post-transplant stage. Evidence from two small prospective randomized trials in cardiac transplant patients and one involving lung transplant recipients showed that resistance training restored BMD toward pretransplantation levels more rapidly and in conjunction with alendronate, was more effective that alendronate alone.
An exercise program, if possible, is helpful in the post-transplant patient.
Pretransplant medication optimization and risk factor modification are important strategies while patients await the procedure. Adjustment and whenever possible replacement of osseous-wasting medications, such as loop diuretics for thiazides and glucocorticoid dose reduction, should be considered (see Box 41-1 ). Patients should be advised about avoidance of alcoholic beverages and they should eliminate any tobacco use.
BONE DISEASE FOLLOWING RENAL TRANSPLANTATION
Renal osteodystrophy, the skeletal complication of end-stage renal disease, is a multifactorial disorder with complex manifestations. The constellation of findings includes those due to secondary hyperparathyroidism (osteoclastic bone resorption leading to subperiosteal resorption and brown tumors), osteosclerosis, osteomalacia, osteoporosis, and soft tissue and vascular calcification). As management of chronic renal disease continues to improve, the prevalence of radiologic changes of renal osteodystrophy is decreasing. For example, prior to the 1970s, when vitamin D metabolism was less well understood, deficiency in 1,25(OH) 2 D due to renal insufficiency, which leads to defective mineralization of osteoid, was a common cause of rickets in the immature skeleton and osteomalacia in the mature skeleton. With improved vitamin D compounds, rickets and severe osteomalacia are now seen less frequently.
Renal osteodystrophy refers to the bone disease seen in patients with chronic renal failure and consists of hyperparathyroidism, osteomalacia, osteosclerosis, osteoporosis, and soft tissue and vascular calcification.
Renal transplantation has become an effective treatment modality for end-stage renal disease and largely restores renal function. Consequently, some of the characteristic findings of renal osteodystrophy may no longer be seen in the post-transplant population, these patients experience persistent abnormalities of mineral metabolism. In addition, pretransplant renal osteodystrophy can significantly contribute to the maintenance or development of post-transplant alterations of bone remodeling. Also, due to glucocorticoid therapy as well as other immunosuppressive drugs such as cyclosporine, tacrolimus, azathioprine, and rapamycin, renal transplant recipients are at significantly increased risk for a wide range of post-transplant bone complications.
Persistent Hyperparathyroidism
Secondary hyperparathyroidism is associated with increased bone turnover and decreased overall bone density. Excess parathyroid hormone affects the ratio of osteoclasts, osteoblasts, and osteocytes, leading to increased osteoclastic bone resorption. After successful kidney transplantation, biochemical evidence of hyperparathyroidism persists in approximately 30% to 50% of recipients. The increases in PTH may be due in part to a large glandular mass of parathyroid cells that develops during chronic renal disease and persists after renal transplantation (tertiary hyperparathyroidism).
Tertiary hyperparathyroidism is characterized by relatively autonomous parathyroid function and hypercalcemia that may occur in patients with chronic renal failure and longstanding secondary hyperparathyroidism.
As in native kidney disease, some patients may develop de novo secondary hyperparathyroidism after kidney transplantation resulting from progressive functional decline of the transplanted kidney. Persistent elevations in the levels of PTH have also been associated with a longer duration of dialysis prior to transplantion. Increased serum levels of PTH are regularly found until 6 months after renal transplantation. After 1 year, levels greater than twice the normal are present in more than 50% of patients, and after 2 years in 27% of patients. Approximately 5% of these patients will require parathyroidectomy to alleviate the symptoms of hyperparathyroidism.
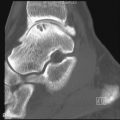
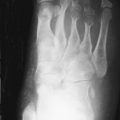
The most common radiographic feature of hyperparathyroidism is bone resorption. The resorption occurs under the periosteum, or involves the cortex, subchondral bone, trabecular bone, endosteum, or ligament insertions. These erosions usually occur at characteristic locations: juxta-articular, tendon/ligament insertions, skull vault, outer ends of clavicles, metaphysis, iliac margins of the sacroiliac joints, and most often, in the hands along the radial margins of the middle phalanges of the index and middle fingers. Erosions of the terminal phalanges (acro-osteolysis) may also occur.
Subperiosteal bone erosion along the radial aspects of the index- and middle-finger middle phalanges and erosion of the cortices of the terminal tufts are characteristic features of hyperparathyroidism.
Osteoclastomas or brown tumors are caused by excessive osteoclastic resorption and localized replacement of bone by vascularized fibrous tissue. Cystic changes may occur due to necrosis. Parathyroid hormone stimulation of osteoblasts may result in periosteal new bone formation. Hyperparathyroidism also leads to osteosclerosis caused by excess accumulation of poorly mineralized osteoid or due to an exaggerated osteoblastic response following bone resorption. Increase in bone density is usually localized to the axial skeleton where the mid-portion of the vertebral bodies are normal in density while the endplates become sclerotic, producing an appearance termed the “rugger-jersey” spine. These radiologic findings of hyperparathyroidism are typical of pretransplant renal osteodystrophy but can be seen in post-transplant patients as well. Periostitis is a rare finding that is most commonly associated with severe disease. This can be seen paralleling the humeri, femurs, tibiae, radii, ulnae, metacarpals, metatarsals, phalanges, and the pubic rami along the iliopectineal line.
Additionally and most importantly, elevated rates of bone turnover and excessive bone resorption due to secondary hyperparathyroidism in the post-transplant patient may be one of several important contributing factors to progressive loss of bone mass and increased fracture risk.
Osteoporosis
Trabecular bone biopsies of post-transplant patients reveal many factors contributing to decreased BMD and subsequent increased fracture risk. Of the spectrum of metabolic bone abnormalities, osteoporosis is one of the most prominent findings in post-transplant patients.
Osteoporosis is defined by the World Health Organization as a progressive systemic disease characterized by low bone density and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture.
Estimated rates of bone loss are reported of up to 6.8% during the first year after transplantation. While some studies suggest this early rapid bone loss is a transient phenomenon, more recent data suggest bone loss continues after the first transplant year with ongoing bone loss found in up to 88% of long-term renal transplant patients with stable renal function. Rapid bone loss within the first 6 to 12 months following renal transplantation is thought to be due primarily to corticosteroid use and the resulting low bone turnover. Immunosuppressive agents used to prevent rejection including cyclosporine A and tacrolimus are now believed to be important risk factors for osteoporosis as well. Both these drugs are shown to cause high-turnover bone loss in animal studies thought to be mediated by cytokines IL-1 and IL-6, which play a major role in the bone resorptive process.
In the initial post-transplant period, when doses of glucocorticoids are high, the corticosteroid effect may predominate, leading to a low bone turnover state. After the first year, maintenance glucocorticoids are relatively low which may allow the high-turnover state induced by cyclosporine or tacrolimus to predominate.
Decreased BMD places transplant patients at a high risk for fractures particularly involving vertebral bodies, ribs and hips, areas rich in trabecular bone. It is estimated that approximately 44% of patients who are at least 5 years post-transplant suffer from osteoporosis-related fractures. Relative to patients with impaired renal function undergoing dialysis, renal transplantation is associated with a 34% greater risk of hip fracture up to 630 days following the transplant.
Renal transplant patients are at risk for osteoporotic fractures in areas of predominantly trabecular bone, such as the vertebral bodies, ribs, and hips.
The spine and proximal femur are two sites rich in trabecular bone and, therefore, most at risk for osteoporotic fracture. Radiographically, osteoporotic bone demonstrates decreased density with relatively thin cortices and a reduced number of trabeculae. In the spine, there is predominant loss of transverse trabeculae and prominence of vertical trabeculae producing a striated appearance. Vertebral deformities are described as biconcave, crush or wedge deformity. These fractures may be detected on radiographs, however, their chronicity may be difficult to determine. Magnetic resonance imaging (MRI) with fluid-sensitive sequences can help differentiate acute from chronic osteoporotic fractures, with acute fractures demonstrating bone marrow edema on fluid-sensitive sequences and increased signal on diffusion-weighted sequences if obtained.
Acute osteoporotic fractures will exhibit edema signal on fluid-sensitive MRI sequences.
Similarly, if radiographs are indeterminate in cases of suspected hip fracture, MRI is a useful next step. Hip fractures that are occult on radiographs, may demonstrate a linear low signal fracture line on T1 or proton-density MRI sequences with accompanying bone marrow edema on fluid-sensitive sequences indicating acute fracture. If MRI cannot be obtained, radionuclide scintigraphy can be diagnostic by demonstrating increased radiotracer uptake in the area of suspected fracture.
Quantitative measurement of BMD is recommended to assess patient risk and plan any necessary treatment. Dual-energy x-ray absorptiometry (DXA scanning) can be performed in clinically relevant sites such as the lumbar spine, proximal femur, and forearm. Quantitative computed tomography (QCT) is a technology that is most often performed for assessment of spine density. The National Kidney Foundation recommends DXA scanning at the time of renal transplantation and at 6 months, 1 year, and 2 years post-transplant. In patients with a “rugger jersey” pattern of vertebral sclerosis from renal osteodystrophy, examination of the spine may be of limited benefit.
DXA scanning is recommended at the time of renal transplantation and at 6 months, 1 year, and 2 years after renal transplantation.
In patients with significantly low bone-mineral density, antiresorptive drugs including bisphosphonates or calcitonin have been suggested depending on the clinical picture and renal function. There is concern that bisphosphonates may lead to adynamic bone disease, however, and some authors use them only for the first postoperative year. Low doses of active vitamin D derivatives and calcium via calcium carbonate have also been shown to prevent bone loss in the lumbar spine and proximal femur when given for the first 6 months after transplantation. Other preventive measures to reduce the risk of osteoporotic fracture include the use of low corticosteroid doses for a limited time in the pretransplant period in patients with end-stage renal disease (ESRD) awaiting transplantation, 30 minutes of vigorous daily physical exercise, limiting alcohol or nicotine consumption, and treating non–transplant-associated risk factors for osteoporosis.
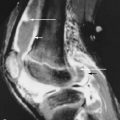
Post-Transplant, Distal-Limb, Bone-Marrow Edema Syndrome
Debilitating musculoskeletal pain is seen in up to one-third of patients following renal transplantation. Osteoporosis related fractures caused by immunosuppressive therapy with corticosteroids have been recognized as the most common causative factor producing bone pain after transplantation.
Debilitating musculoskeletal pain is seen in up to one-third of patients following renal transplantation.
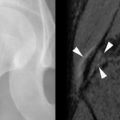
In the last decade, however, an unusual pain syndrome in the lower limbs has been reported with increasing frequency. Symptoms are characterized by episodes of severe bilateral distal lower limb pain occurring within the first 2 years after transplantation with most episodes occurring in the first several months after surgery and persisting for weeks to months. Patients describe worsening pain with physical stress such as walking. Patients also have elevation in their serum alkaline phosphatase. The first description of this entity relating the pain syndrome to cyclosporine therapy in renal-transplant recipients was reported by Lucas et al. in 1991. Since then, the symptoms have been found most commonly in patients treated with cyclosporine and tacrolimus, and the syndrome is frequently referred to as calcineurin-inhibitor–induced pain syndrome (CIPS). However, recently cases have been reported in patients not on calcineurin inhibitors leading to renaming of the condition as post-transplant, distal-limb, bone marrow edema syndrome. While the pathogenesis is still uncertain, post-transplant, distal-limb bone marrow edema is often associated with high calcineurin-inhibitor levels and reduction of calcineurin-inhibitor doses or other immunosuppressive drugs has been shown to alleviate symptoms. One hypothesis points to a calcineurin-inhibitor–induced vasculitis leading to increased bone perfusion and permeability, ultimately resulting in bone marrow edema and pain. Microscopic fractures with marrow edema and alterations of pain threshold have also been suggested as possible etiologies.
CIPS (calcineurin-inhibitor–induced pain syndrome), also called “post-transplant, distal-limb, bone marrow edema syndrome” refers to bone pain that occurs in the distal lower limbs of patients after transplantation.
Radiographic findings of post-transplant, distal-limb, bone marrow edema syndrome include a normal initial appearance or nonspecific diffuse or patchy osteoporosis. Bone scintigraphy demonstrates increased radiotracer uptake on all three phases of the bone scan with an unusually symmetric accumulation of tracer on the late phase in the clinically affected areas. MRI findings can be striking with patchy bone marrow edema (poorly defined low-signal intensity on T1-weighted MRI sequences and high-signal intensity on T2-weighted sequences), which can include the entire involved bone, and may extend from the epiphyseal to the metaphyseal regions of long bones. Insufficiency fractures (low-signal lines within the edematous regions) may also be present ( Figure 41-2 ).