Bone Formation and Growth
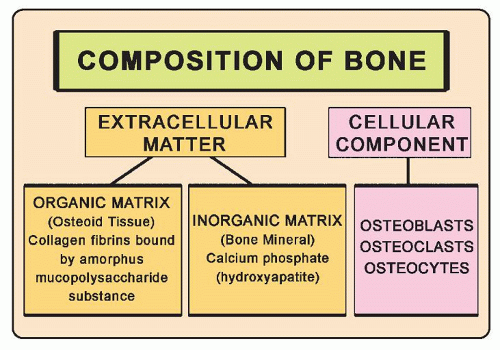
The skeleton is made of cortical and cancellous bones, which are highly specialized forms of connective tissue. Each type of bony tissue has the same basic histologic structure (Fig. 3.1), but the cortical component has a solid, compact architecture interrupted only by narrow canals containing blood vessels (haversian systems), while the cancellous component consists of trabeculae separated by fatty or hematopoietic marrow. A bone is a rigid calcified material and grows by the addition of new tissues to existing surfaces. The removal of unwanted bones, called simultaneous remodeling, is also a necessary component of skeletal growth. Unlike most tissues, a bone grows only by apposition on the surface of an already existing substrate, such as a bone or calcified cartilage. Cartilages, however, grow by interstitial cellular proliferation and matrix formation.
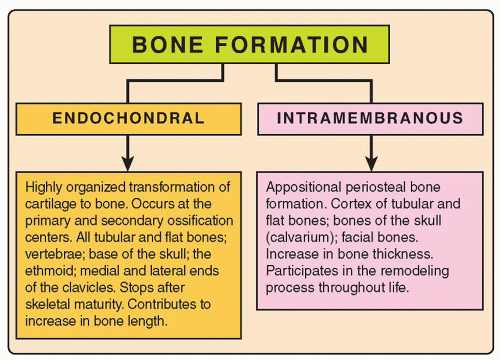
A normal bone is formed through a combination of two processes: endochondral (enchondral) ossification and intramembranous (membranous) ossification (Fig. 3.2). In general, the spongiosa develops by endochondral ossification and the cortex by intramembranous ossification. Once formed, a living bone is never metabolically at rest. Beginning in the fetal period, it constantly remodels and reappropriates its minerals along lines of mechanical stress. This process continues throughout life, accelerating during infancy and adolescence. The factors controlling bone formation and resorption are still not well understood, but one fact is clear: Bone formation and bone resorption are exquisitely balanced, coupled processes that result in net bone formation equaling net bone resorption.
Most of the skeleton is formed by endochondral ossification (Fig. 3.3), a highly organized process that transforms a cartilage to a bone and contributes mainly to increasing the bone length. Endochondral ossification is responsible for the formation of all tubular and flat bones, vertebrae, the base of the skull, the ethmoid, and the medial and lateral ends of the clavicle. For example, at approximately 7 weeks of embryonic life, cartilage cells (chondroblasts and chondrocytes) produce a hyaline cartilage model of the long tubular bones from the condensed mesenchymal aggregate. The mechanisms leading to the calcification of the cartilaginous matrix are
not completely understood, but it is generally believed that the promoters of calcification are small membrane-bound vesicles known as matrix vesicles, which are present in the interstitial matrix between the cells. At approximately the 9th week, peripheral capillaries penetrate the model, inducing the formation of osteoblasts. Osseous tissue is then deposited on the spicules of the calcified cartilage matrix that remain after osteoclastic resorption, thereby transforming the primary spongiosa into the secondary spongiosa.
not completely understood, but it is generally believed that the promoters of calcification are small membrane-bound vesicles known as matrix vesicles, which are present in the interstitial matrix between the cells. At approximately the 9th week, peripheral capillaries penetrate the model, inducing the formation of osteoblasts. Osseous tissue is then deposited on the spicules of the calcified cartilage matrix that remain after osteoclastic resorption, thereby transforming the primary spongiosa into the secondary spongiosa.
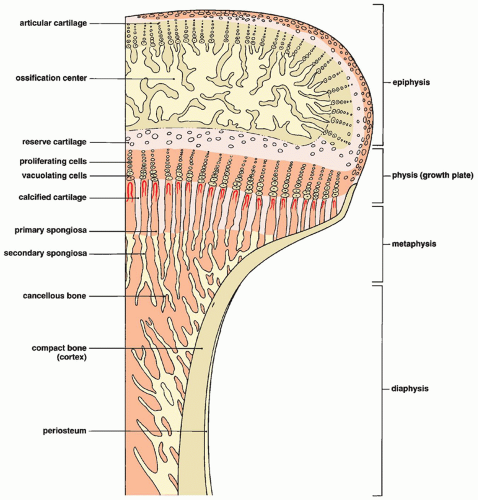
As this process moves rapidly toward the epiphyseal ends of the cartilage model, a loose network of bony trabeculae containing cores of calcified cartilage is left behind, creating a well-defined line of advance. This line represents the growth plate (physis) (Fig. 3.4) and the adjacent metaphysis to which the secondary spongiosa moves as it is formed. The many trabeculae of the secondary spongiosa that are resorbed soon after being formed become the marrow cavity, while other trabeculae enlarge and thicken through the apposition of a new bone, although these too eventually undergo resorption and remodeling. Others extend toward the shaft and become incorporated into the developing cortex of the bone, which is formed by intramembranous ossification. At the ends of tubular bones, a similar process is initiated, creating a secondary ossification center in the epiphysis. This nucleus increases in size by the process of maturation and calcification of the cartilage surrounding the secondary center. The peripheral margin of epiphysis termed acrophysis is formed of zones of cell hypertrophy, degeneration, calcification, and ossification, similar to that of the growth plate. Endochondral bone formation is not normally observed after growth plate closure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree