Jamshid Tehranzadeh, MD
OSTEOCHONDRITIS DISSECANS
Osteochondritis dissecans (OCD) is a poorly characterized process that involves a fragmentation of articular cartilage and/or subchondral bone resulting in focal devitalization that can result in debilitating pain, inflammation, and degeneration. If left untreated, end-stage arthritis may occur as a result of abnormal wear patterns and continued cartilaginous injury.
Originally thought to be the primary result of inflammation, the condition was termed “osteochondritis”; although, its true underlying cause is not known, trauma appears to be a predisposing factor. Other contributing causes may include genetic predisposition, ischemia, or ligamentous abnormality/laxity. “Dissecans” refers to the Latin root “to dissect” and is reflected by the separation of cartilage and subchondral bone.
 Pathology
Pathology
At the cartilage-bone interface, chondrocytes form articular cartilage, which is composed of hydrated proteoglycan and collagen. In OCD, it is thought that damage to this protective layer of proteoglycans results in disintegration and desiccation of the cartilage. Cartilage lacks innervation; however, progressive damage results in exposure of the underlying bone, which causes pain and disability. Thus, by the time that the disorder becomes clinically apparent, significant cartilaginous damage has already occurred.1
OCD affects children as well as adults. The incidence of juvenile OCD, in which the physis is unfused, is increasing. This is likely owing to participation in competitive sports, especially those involving jumping and axial loading. Males represent the majority of patients with juvenile OCD, although the incidence among females is also rising. Adult OCD is unusual over the age of 50 years, although it can affect patients of any age. It is unclear if adult OCD results from prior/juvenile injury, although de novo adult cases have been reported. There is likely some overlap in the two forms of the disease, although the general distinction remains the presence (or absence) of an open physis. Chondrocytes have very little capacity for regeneration, although in the juvenile form of the disease, approximately 50% will heal, whereas in the adult form, very few will heal without intervention and often progress to osteoarthritis.
 Anatomy
Anatomy
The knee (Figure 9-1) is the most commonly affected joint, representing approximately 75% of the cases, although other joints including the ankle (Figure 9-2) and elbow are also commonly seen. Rarely, OCD can present in the shoulder, hand, wrist, or hip. The defect is usually seen in a single joint, although it is bilateral in approximately 20–30% of cases.
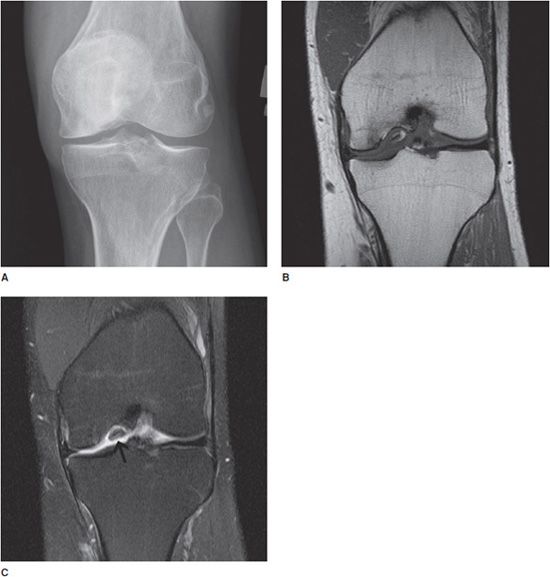
Figure 9-1. Osteochondritis dissecans (OCD) of the medial femoral condyle. AP view (A) of the knee shows flattening and sclerosis of the medial femoral condyle. There is minimal associated joint space narrowing. Coronal T1 image (B) of the same knee demonstrates low T1 signal. Coronal T2 image (C) demonstrates high signal in the medial femoral condyle compatible with edema. A fracture and unstable osteochondral bony fragment (arrow) are noted.
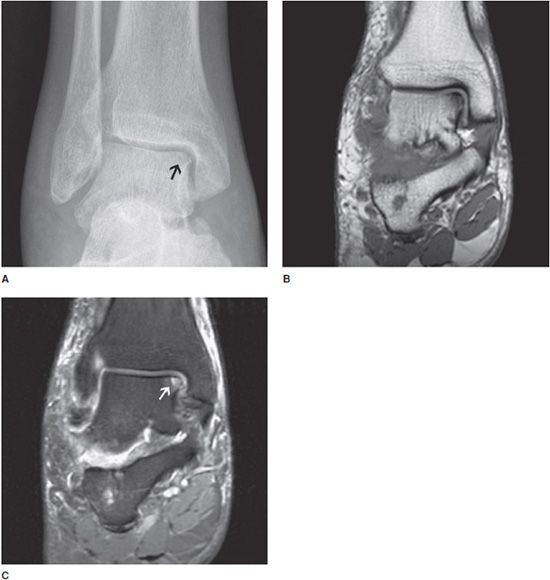
Figure 9-2. Osteochondritis dissecans (OCD) of the medial talus. AP view (A) of the ankle demonstrates mild focal cortical irregularity with associated lucency at the subchondral bone of the medial dome of the talus. Coronal T1 and T2 images (B,C) demonstrate low T1 and high T2 signal in the area of abnormality noted on the radiograph compatible with subchondral edema (arrow) with cortical irregularity.
 Radiological Findings
Radiological Findings
Since its discovery, there have been numerous imaging criteria for the classification of OCD. Most were developed to describe lesions in the knee, as it is the most commonly affected joint. Assessment of the joint is typically with two orthogonal radiographs: in the case of the knee, the anteroposterior (AP) and lateral radiographs. However, other views may prove helpful in the diagnosis and characterization of OCD. The oldest classification system was described by Berndt and Harty in 1959 and was based on radiographs of the talus:
• Stage 1: Focal area of compression of the subchondral bone
• Stage 2: Partially detached osteochondral fragment
• Stage 3: Completely detached, but nondisplaced fragment
• Stage 4: Detached and displaced fragment/loose body within the joint
Other modalities such as Tc99m bone scan may demonstrate focal uptake. MRI has a separate staging classification as follows:
• Stage 1: Subchondral bone flattening or bone marrow edema
• Stage 2A: Subchondral cyst
• Stage 2B: Incomplete separation of the osteochondral fragment
• Stage 3: Effusion (fluid around an undetached, undis-placed osteochondral fragment)
• Stage 4: Complete detachment of osteochondral fragment and formation of loose body
Sensitivity increases with gadolinium arthrogram. This classification system has been correlated with arthroscopic findings, and high-grade (3 or 4) lesions are considered unstable.
PEARLS
OSTEOCHONDROSES
Osteochondrosis is a poorly characterized inhomogeneous group of disorders involving articular cartilage and subchondral bone. Osteochondrosis is basically osteonecrosis of subchondral bone. Initially, the set of disorders and findings associated with osteochondroses were thought to arise from a similar pathology developing from osteonecrosis; however, it is now widely felt that this grouping of disorders shares no definite common pathology and in some cases may represent normal variation. Some sources only consider defects of immature skeletons (those with open physes) as osteochondroses; however, that distinction will not be made in this text. The classification of osteochondrosis is thought to include disorders of osteonecrosis and conditions related to abnormal stresses, although more specific definitive causes remain elusive.
 Osteochondroses Involving Osteonecrosis
Osteochondroses Involving Osteonecrosis
In this section, we will discuss the various presentations and radiographic findings associated with osteonecrosis. When discussing osteonecrosis, it is important to distinguish between juvenile and adult forms of the disorder. Various eponyms have been assigned to some of the most commonly presenting lesions and we will present and discuss several of them here.
 Legg–Calvé–Perthes Disease
Legg–Calvé–Perthes Disease
Legg–Calvé–Perthes disease is characterized by osteonecrosis of the femoral head (Figures 9-3 and 9-4), generally in children between 4 and 8 years old. The cause of the osteonecrosis is hypothesized to include trauma or increased intra-articular pressure; however, no definite cause has been identified. Bilateral involvement is atypical, but does occur. The clinical presentation of Legg–Calvé–Perthes disease includes hip pain, limping and limited range of motion. radiographically, Legg–Calvé–Perthes disease presents with adjacent focal soft tissue swelling. It can present with a tiny femoral epiphyseal ossification center, lateral displacement of the femoral epiphyseal ossification center, fracture, irregularity and sclerosis as well as vacuum phenomenon within the epiphysis. This lesion may be reversible if there is reconstitution of the blood supply to the femoral head, and radiographic abnormalities may also reverse to the point that it is indistinguishable from the uninvolved side.2 The differential diagnosis for femoral head collapse in children is wide and includes systemic abnormalities such as hypothyroidism, sickle cell anemia, and Gaucher disease in addition to infection, eosinophilic granuloma, and epiphyseal dysplasia.
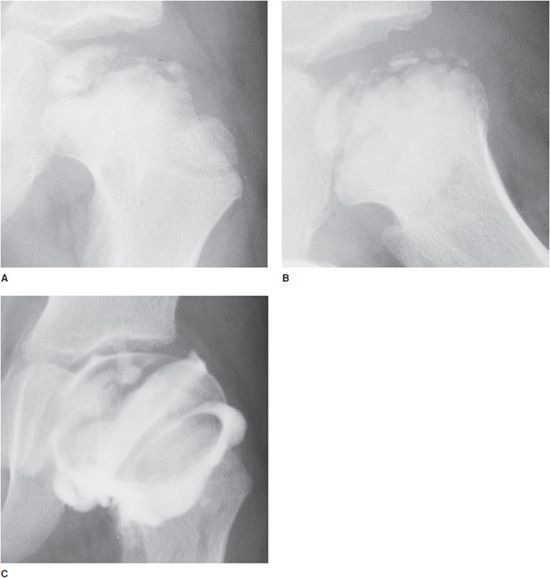
Figure 9-3. Legg–Calvé–Perthes disease (idiopathic AVN). AP view (A) and frog leg (B) lateral radiographs of the left hip in a 6-year-old boy show sclerosis and fragmentation of the femoral head. Note widening of the metaphysis with enlargement of the femoral head (coxa magna). The joint space is also widened due to cartilage hypertrophy. A slight lateral subluxation of the femoral head is also seen. The arthrogram (C) shows uncalcified cartilage that is visualized by injection of contrast agent into the joint.
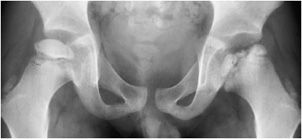
Figure 9-4. Legg–Calvé–Perthes disease. AP view of the pelvis in a boy shows sclerosis, irregularity, and fragmentation of the femoral epiphysis with metaphyseal widening on the left. Milder changes are seen on the right side.
 Freiberg Infraction
Freiberg Infraction
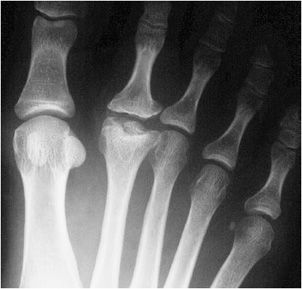
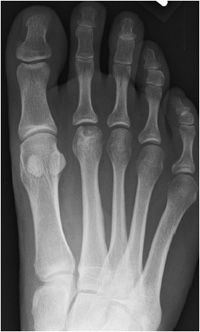
Similarly, in Freiberg infraction, the defect typically occurs in the second metatarsal head (Figures 9-5 and 9-6), although to a lesser extent the third and fourth metatarsal heads may also be involved. It is more commonly seen in females. Its onset is insidious and may result in pain, swelling, necrosis, and functional disability. Radiographically, flattening and sclerosis of the metatarsal head characterize Freiberg infraction with possible cystic regions in addition to widening of the metatarsophalangeal joint.3 This abnormality is typically noted in adolescence, although it can be seen in adults as well. The features of Freiberg infraction are essentially pathognomonic and no differential diagnosis should be entertained. Staging of Freiberg infraction is through the Smillie system developed in 1967.
Figure 9-5. Freiberg infraction. AP view of the right foot in a 24-year-old woman shows sclerosis and deformity of the second metatarsal head with osteochondral defect indicating osteochondrosis.
Figure 9-6. Freiberg infraction. AP view of the foot in a 34-year-old woman shows sclerosis and lucency of the second metatarsal head with osteochondral abnormality indicating osteochondrosis.
 Kienböck Disease
Kienböck Disease
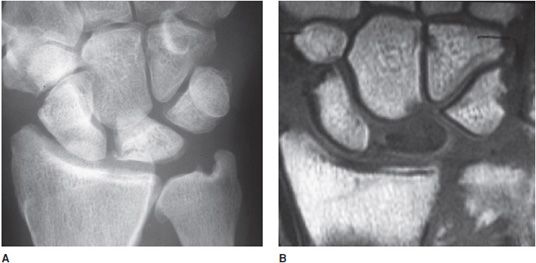
Kienböck disease is another eponymous disorder assigned to osteonecrosis of the lunate within the wrist (Figure 9-7). Traditionally, the disease presents in young adults between 20 and 40 years of age.4 It is generally accepted that the cause of Kienböck disease is multifactorial with the end-result being osteonecrosis of the lunate. MRI is more sensitive to the presence of Kienböck disease than radiographs, especially with early disease, making MRI an ideal imaging modality for this abnormality. A four-stage imaging classification system is used to grade Kienböck disease (Lichtman four-stage classification system):
Figure 9-7. Kienböck disease. AP radiograph (A) of the wrist shows sclerosis and fracture and collapse of the lunate bone indicating AVN. T1-weighted spin-echo image (B) of the wrist shows decreased signal of the lunate bone with collapse indicating AVN with fracture.
• Stage I: Normal radiographic findings with homogenous low signal on T1- and T2-weighted MRI.
• Stage II: Definite density changes of the lunate on radiograph (relative to the other carpal bones) without abnormal size, shape, or position of the lunate.
• Stage III: Disrupted carpal architecture and collapse of the lunate with subluxation of the capitate proximally.
• Stage IV: Radiocarpal and intercarpal degenerative changes in the setting of stage III findings.
 Kohler Disease
Kohler Disease
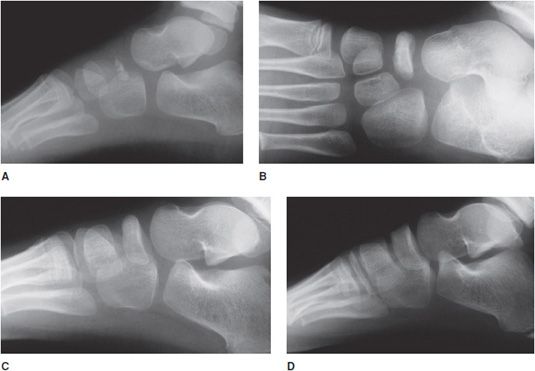
Kohler disease is a radiographic normal variant and the affected children outgrow this apparent abnormality. This is a relatively rare entity involving sclerosis of the navicular bone in the foot. The majority of cases are unilateral presenting in children between 3 and 7 years of age. Early in the course of the disease, radiographs may demonstrate patchy sclerosis and fragmentation of the navicular with adjacent soft tissue swelling (Figure 9-8A). The osseous structure may be flattened, irregular, and diminutive; however, the surrounding interosseous space may be maintained (Figures 9-8B,C). Over several years, the changes may reverse themselves such that the navicular may appear normal (Figure 9-8D).
Figure 9-8. Kohler disease. (A) Lateral view of the foot in a 3-year-old boy shows sclerosis of the partially ossified tarsal-navicular bone that mimics osteochondrosis (AVN). (B) One year later (age 4), the tarsal-navicular is more ossified, but still remains partially sclerotic and may mimic AVN of the tarsal navicular bone. (C) Two years after initial study (age 5), the examination shows normal tarsal navicular with resolution of previously noted sclerosis. (D) Two years later (age 7), the tarsal navicular appears normal.
 Panner Disease
Panner Disease
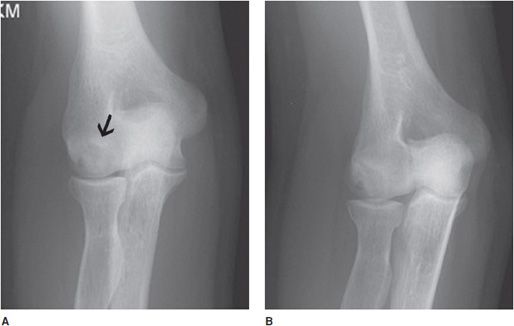
Panner disease is a rare osteochondrosis of the humeral capitellum typically affecting boys between 5 and 10 years of age. Panner disease may be associated with pediatric baseball players. Clinically, this entity can be very painful and may present with decreased range of motion, focal tenderness at the lateral condyle, and splinting. The etiology of Panner disease, as with most of the osteochondroses, is not well understood, although it is generally accepted to relate to excessive or repetitive valgus stress at the elbow joint at a point in skeletal growth in which there is a vulnerable blood supply to the capitellum. After age 5, there are only 1 or 2 end arteries serving the capitellum, whereas in younger patients, there is a rich vascular bed. By the late teenage years, a network of numerous metaphyseal anastomoses feeds the capitellum, protecting against avascular necrosis (and thus, Panner disease).
Radiographs most frequently demonstrate cortical irregularity and capitellar osteopenia, although fragmentation, resorption, and/or diminutive size are also commonly seen (Figure 9-9). Like Kienböck disease, MRI is more sensitive in detecting early bone marrow changes and may demonstrate abnormalities without any radiographic changes. Early diagnosis is important to identify and cease the offending activity. As with other osteochondroses, Panner disease is known to reverse itself. The differential diagnosis includes OCD of the elbow, with the differentiating feature being the age of the patient. However, it should be noted that some sources consider Panner disease and OCD of the capitellum to be within the same spectrum of disease.5
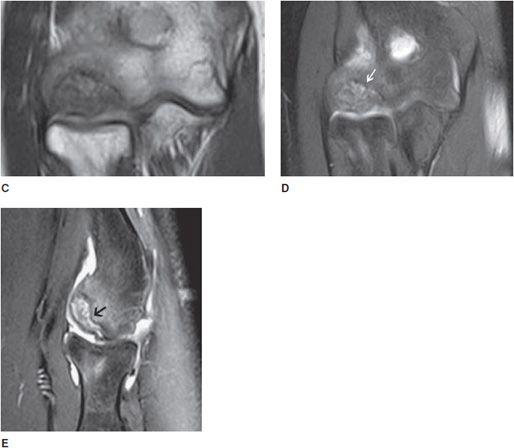
Figure 9-9. Osteochondrosis of the capitellum (Panner disease). AP view and oblique (A,B) radiographs of the elbow show subchondral sclerosis and lucency (arrow in A) with cortical irregularity of the capitellum. Coronal T1-weighted (C) image shows subchondral low signal intensity, and the coronal and sagittal fat-saturated T2 (D,E) images show subchondral mixed bright signal intensity, with cortical irregularity due to osteochondral defect (osteochondrosis) of the capitellum, so-called Panner disease (arrow in D).
 Scheuermann Disease
Scheuermann Disease
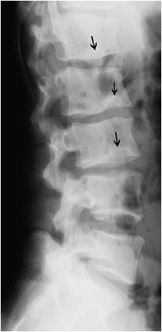
Scheuermann disease is osteonecrosis of the ring epiphysis of the vertebral column. It is a cause of juvenile kyphosis and back pain. Radiographically, Scheuermann disease presents as irregularity of the vertebral endplates and wedge deformity of vertebral bodies (Figure 9-10). Softened endplates may result in invagination of disc material (Schmorl nodes). Generally, multiple endplates are involved. Treatment is controversial and may include conservative therapy, bracing, or surgery depending on the degree of disability and pain.
Figure 9-10. Scheuermann disease. Lateral view of the lumbar spine in a 20-year-old man shows irregularity of the endplates of the vertebral bodies with multiple Schmorl nodes (arrows) and slight decrease in height of the vertebral bodies due to the soft vertebral endplates.
 Osteochondroses Involving Trauma
Osteochondroses Involving Trauma
This section of osteochondroses involves the diseases that are generally associated with a prior history of trauma, either a single insult or repetitive stresses. As with the entities involving osteonecrosis, this section will again be peppered with eponyms.
 Osgood–Schlatter Disease
Osgood–Schlatter Disease
Osgood–Schlatter disease is a disorder involving fragmentation and soft tissue swelling of the tibial tuberosity, typically presenting in early teenage boys. It can be bilateral in approximately 25% of the cases. It is considered to be one of the more common causes of knee pain in children. Acutely, there is prominent soft tissue swelling, which may resolve in the follow-up imaging; however, there is persistent fragmentation. This disease is likely the result of repetitive tensile insult of the insertion of the patellar tendon upon the tibial tuberosity, possibly from a discrepancy in the strength of the quadriceps muscle and the growing bone. The condition is related to activities involving running and jumping and is observed in about 20% of adolescents who participate in such activities. It is important to keep in mind that fragmentation of the tibial tuberosity without focal soft tissue swelling or pain may occur in the absence of Osgood–Schlatter disease, and is recognized as a normal variant. MRI may demonstrate partial cartilaginous avulsion of the tibial tubercle prior to any radiographic findings and may be atypical in presentations.
Accurate diagnosis of Osgood–Schlatter disease requires both astute radiological findings and clinical symptoms. Clinically, there may be focal tenderness at the tibial tuberosity or pain with knee extension. It is widely accepted that the large majority of patients diagnosed with Osgood–Schlatter disease will fully recover. However, a minority will continue to have symptoms into adulthood. The differential diagnosis includes Sinding–Larsen–Johansson syndrome, which is similar but occurs in a separate anatomic location (as discussed in the following section). Hoffa syndrome, in which there is localized injury of the infrapatellar fat pad, may present clinically similar; however, there is no osseous or cartilaginous injury. Tibial tubercle fracture may also present similarly, and the lateral radiograph of the knee should be thoroughly evaluated for a fracture line.6
 Sinding–Larsen–Johansson Syndrome
Sinding–Larsen–Johansson Syndrome
A corollary disease to Osgood–Schlatter is Sinding-Larsen– Johansson syndrome in which tensile forces at the inferior pole of the patella at the insertion of the patellar tendon result in tendinous thickening and calcification at the inferior patellar pole resulting in tenderness and soft tissue swelling. Fragmentation of the inferior patella is occasionally seen with this entity; however, this finding alone may represent a normal variant.
 Blount Disease
Blount Disease
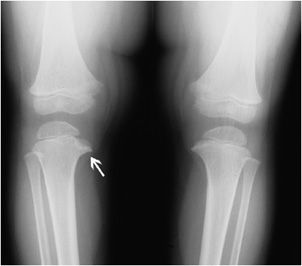
Blount disease is also known as osteochondrosis deformans tibiae and tibia vara deformity. It was initially described as tibial varus deformity with beaking of the medial tibial metaphysis, deformity of the medial epiphysis, widening of the growth plate, and cartilaginous islands adjacent to the tibial beak (Figure 9-11). Tibial varus deformity is defined as medial bowing of the tibial metaphysis and diaphysis as the bone descends. The disease is uncommon, typically presenting in African-American children between the ages of 6 and 13. As the child develops and gains weight, the varus deformity worsens resulting in knee pain and, if severe, restricted mobility. In addition to excess weight, abnormal joint laxity and genetic predisposition are suggested as contributing factors. The differential diagnosis includes physiologic bowing, although it tends to be more severe in Blount disease and results from abnormalities within the proximal tibia rather than angulation at the tibiofemoral joint. Additionally, a diagnosis of healing or mild rickets disease may be entertained in the setting of lower extremity bowing.7
Figure 9-11. Blount disease. AP view of both knees in a boy showing mild genu varus deformity (bowlegged) with hypertrophic irregular medial tibial metaphyseal osteophytes (arrow) and deformity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree