Muscles
Units of onabotulinumtoxinA (Botox)
Lumbar paraspinal muscles
5–50 units bilaterally at each vertebral level (includes the multifidi and the lumbar iliocostalis muscles)
Psoas muscle
50–100 units
Quadratus lumborum
25 units
There have been several studies that have shown a beneficial effect of botulinum toxin on low back pain. The studies were done on individuals with chronic low back pain that was predominantly unilateral and who did not have an identifiable acute source of pain like a herniated disc. Back pain in this sense can be called nonspecific low back pain. Foster et al. [14] evaluated the effect of botulinum toxin A over 8 weeks on nonspecific low back pain in a randomized, double-blind study. Botulinum toxin type A 40 units or saline was injected unilaterally at five different lumbar levels. Relief was greater than 50 % (VAS score) in 73 % of the botulinum toxin type A-treated subjects compared to 25 % of the saline control group (p = 0.012). A follow-up open-label study by the same group was conducted over 14 months. The outcomes in the open-label extension of the study included the change in the VAS, the number of pain days, and the Oswestry functional status scale. Botulinum toxin type A was found to significantly reduce pain over a 14-month period of time during which repeated injections were given. Ninety-one percent of initial responders maintained responsiveness over the duration of the open-label trial [15].
Neck and Upper Back Pain (Table 35.2)
Table 35.2
Injection dosing schedule recommended for chronic, intractable neck pain
Muscle | Units of BoNT-A |
|---|---|
Upper trapezius | 15–40 units distributed through the muscle |
Splenius capitis | 5–10 units |
Splenius cervicis | 5 units |
Semispinalis | 5–10 units as 5 units in each of 1–2 units sites |
Oblique capitis inferior | 5 units |
Levator scapulae | 25 units |
Medial scalene | 5 units |
There are few randomized, controlled studies of the use of BoNT in neck and upper back pain. Göbel et al. [16] found that BoNT-A (Dysport®) significantly reduced or eliminated upper back myofascial pain compared to saline (51 vs. 26 %, p = 0.002) 5 weeks after injection. However, another study of BoNT-A treatment of pain emanating from cervicothoracic myofascial TrPs showed that low dose (50 units total), higher dose (100 units total), and saline all resulted in a decline in pain and disability scores, with no benefit of BoNT over placebo, but those in the subgroup of subjects who received a second injection of BoNT-A experienced such a high incidence of becoming asymptomatic that the authors concluded that further studies were warranted. In contrast, a RCT, double-blinded study of the effect of BoNT-A in the treatment of refractory neck pain, in which 33 of 47 subjects had neck injury (72 %), resulted in a significant number of subjects classified as excellent responders who had a ≥50 % decrease in VAS for pain, ≥30 % reduction in pain days, and 2 grades or more improvement in the Modified Oswestry Pain Questionnaire (6/23 in the BoNT-A group vs.1/21 in the control group). Pain intensity was greater in the BoNT-A group at baseline, but was significantly decreased at 2 months compared to the saline control group (p < 0.0018). Injections were made in the trapezius, splenius, and rhomboid muscles as clinically indicated, using 20 units per injection site and ranged in total from 150 to 300 units. No information was given about the criteria for selection of specific injection sites, but it appears that the injection sites were selected to give a general distribution of BoNT-A in the muscles treated, as opposed to injecting in myofascial trigger points. This well-designed and executed study clearly shows efficacy of BoNT-A treatment for chronic neck pain.
Myofascial Pain Syndromes
Pain in myofascial pain syndrome (MPS) is generated by a tender, taut band that is the fundamental feature of the myofascial trigger point (MTrP). It is thought that relaxation of the taut band through inhibition of acetylcholine release from the motor nerve terminal at the neuromuscular generation and inhibition of the release of neurotransmitters at the sensory nerve will result in decreased pain from MTrPs. However, the few studies that have looked at this have not supported this concept. Often-quoted studies include those of Ferrante et al. [17], Graboski et al. [18], Kamanli et al. [19], and Ojala et al. [20]. Ferrante et al. [17] stopped all pain medication prior to the trial injections, then gave all subjects amitriptyline, ibuprofen 800 mg qid, and prn propoxyphene/apap. In this RCT, subjects were given either BoNT-A (10, 25, or 50 units/trigger point) or saline in up to 5 trigger points. Patients were excluded if they had more than 5 trigger points or more than 2 trigger points in on trapezius muscle, or more than one trigger point in any other single surface muscle. There was no difference in rescue medication, or in pressure pain threshold between saline injected groups and those injected with BoNT-A. Both the lowest dose and highest dose BoNT-A outperformed saline, but not to a significant degree. Some of the possibilities include the fact that all subjects were treated with adjuvant pain medication and that saline is not an inactive control. Finally, the standard deviation of the sum of pain intensity differences, a reflection of the primary outcome measure, was so great that differences between the saline group and the BoNT-A were not significant. For these reasons, the results of this study cannot be seen as conclusive. Graboski et al. [18] compared BoNT-A to bupivicaine. A maximum of 8 trigger points were injected with either 25 units of BoNT-A or saline in this RCT. The end point was a return to 75 % of pre-injection pain. At that time, subjects were injected with the other substance (a crossover study). There was no statistically significant difference between the two treatments. Bupivicaine was considered cheaper. Outcome measures were changed in pain scores and duration of relief. Although there was no significant difference between groups, there was a definite trend toward greater decrease in pain and greater duration of effect with BoNT-A. Possible issues were the low number of subjects completing the trial (17 subjects in all) and the limited number of trigger points injected. Kamanli et al. [19] compared BoNT-A, lidocaine, and dry needling. Pain scores were lower in the subjects treated with BoNT-A and dry needling, and visual analogue scale significantly decreased in the lidocaine and BoNT-A groups at the single follow-up visit of 4 weeks. A single trigger point was treated. BoNT-A was not inferior to lidocaine or dry needling. However, in this study, as well as in the other two studies, no attempt was made to provide a comprehensive treatment to clear all relevant trigger points. In clinical practice, one would treat all of the trigger points deemed to be relevant in producing a pain syndrome. In this regard, inactive trigger points that are known to cause dysfunction [21] also need to be treated. In partial treatments, trigger points tend to recur more quickly. Studies that use pressure pain thresholds as a measure have not shown that this measurement reliably reflects inactivation of trigger points.
Ojala et al. [20] did a single-blind, RCT, crossover design, injecting either saline or BoNT-A in up to six neck and shoulder muscles bilaterally and then injecting the trigger points with the other substance 1 month later. There was no difference between groups 1 month after the second injection when subjects had received both saline and BoNT-A 1 month apart. However, after the first month, before the saline group received BoNT-A, subjects treated with BoNT-A had a significantly greater decrease in pain than those treated with saline. BoNT-A has a peak motor effect at about 6 weeks, and effectiveness is maintained from 10 to 14 weeks in most subjects. Hence, one would expect a difference 1 month after the first injection and no difference 1 month after the crossover injection.
There are a number of other studies that address this issue. Pain in patients with temporomandibular joint disorders was treated with BoNT-A injected into the masseter and temporalis muscles in a randomized, controlled study [22]. The result was a decrease in pain and an improvement in psychological status. Both the studies of Göbel et al. [16] and Wheeler et al. [23] were suggestive of a benefit for BoNT at a certain time and under certain circumstances.
It helps to be very clear in defining the desired outcome using BoNT. All studies show that BoNT treatment of myofascial trigger points can respond well to BoNT, but the placebo effect has also been striking. It is essentially like giving a lidocaine trigger point injection that lasts between 10 and 14 weeks in duration. The local twitch response that marks a successful trigger point injection with lidocaine is generally lost after BoNT injection. The number of lidocaine trigger point injections needed to treat an MPS is usually reduced and sometimes eliminated altogether for the duration of action of the toxin. The effectiveness of the toxin in MPS seems to depend on the care with which the trigger point zone is injected and the comprehensive clearing of TrPs in a functional muscle unit. Treatment of trigger points in only one muscle instead of a functional muscle unit may be a reason why spontaneous pain was not reduced 28 days after BoNT-A was injected in an infraspinatus muscle trigger point [24].
BoNT in Headache Management
Migraine Headache (Tables 35.3, 35.4, and 35.5)
Table 35.3
Treatment of migraine headache by Botox: migraine protocol
Muscle | Units of onabotulinumtoxinA (Botox) |
|---|---|
Temporalis | 25 in a linear or diamond grid |
Procerus | 5 |
Corrugator | 5 each |
Frontalis | 10–20 |
Table 35.4
Follow-the-pain protocol for use in chronic tension-type headache and migraine
Muscle with trigger points referring pain to headache regions | Units of onabotulinumtoxinA (Botox) |
|---|---|
Temporalis | 25 in a linear or diamond grid |
Masseter | 2–4 |
Zygomaticus | 2–3 |
Pterygoid | 2–3 |
Sternocleidomastoid (inject only one side) | 10–15 |
Splenius capitis | 5 |
Splenius cervicis | 5 |
Oblique capitis inferior | 5 |
Semispinalis | 5–10 |
Levator scapulae | 10–25 |
Upper trapezius | 25–40 |
Table 35.5
Combined migraine and follow the pain for most migraine headaches
Muscle | Units of onabotulinumtoxinA (Botox) |
|---|---|
Upper trapezius | 25–40 |
Sternocleidomastoid (inject only one side) | 10–15 |
Temporalis | 25 in a linear or diamond grid |
Procerus | 5 |
Corrugator | 5 |
Splenius capitis and cervicis | 5 |
Semispinalis | 5 |
Oblique capitis inferior | 5 |
The headache literature is very mixed on the issue of the effectiveness of BoNT in the treatment of headache in general, and migraine in particular. This literature was reviewed through 2007/2008 in Gerwin [25]. The literature prior to 2010 can be summarized by saying that the results of the many studies done in patients with migraine and other headaches varied in their outcome. One of the reasons for variability this is the lack of uniformity in subject selection, sites, and dosing schedules used. Silberstein et al. [26], in a RCT of chronic migraine showed that patients treated at predetermined fixed sites with low-dose onabotulinumtoxinA (25 units total) had a significantly reduced migraine frequency and a reduction in the use of medications compared to the carrier alone, but the higher dose of 75 units showed no benefit. A RCT using lower doses of BoNT in the temporalis muscles and omitting injection of glabellar muscles showed a 50 % reduction in headache days in 30 % of the active treatment group, but the 25 % of the placebo group showed the same response, so the results were not significant [27]. A study using three different dosing schedules of BoNT-A and using a low dose (7.5 units) of BoNT as an active control showed equal reduction in headache frequency in all groups [28]. A small study failed to show benefit of BoNT-A in reduction of headache frequency or severity compared to placebo, but headache index worsened in the placebo group, but not in the active treatment group, suggesting that BoNT had a protective effect in headache severity. Studies of episodic migraine fared no better [29, 30]. However, there two studies that indicated that BoNT-A to be of benefit in the treatment of migraine [31, 32]. The largest and most comprehensive RCT, to date, of 1,384 subjects was done by the PREEMPT Chronic Migraine Study Group [33] and found that onabotulinumtoxinA (BoNT-A) was superior to placebo in the primary outcome measure of reduction in frequency of headache days at all time points in the 24-week double-blinded portion of the trial. In addition, onabotulinumtoxinA was superior to placebo in six secondary outcome measures: mean change from baseline in frequencies of headache days, moderate or severe headache days, cumulative hours of headache on headache days, headache episodes, migraine episodes, and the proportion of patients with severe Headache Impact Test-6 score. Acute medication use in the treatment group was not statistically better than placebo. The size of the study and the thoroughness of evaluation make this a landmark study. The authors point out that the study was not made against a comparator. The authors themselves refer to a pilot study comparing onabotulinumtoxinA to topiramate [34], an FDA-approved drug for the prevention of migraine. In this study, subjects were randomly assigned to either onabotulinumtoxinA or to topiramate. The study was placebo controlled.
Technique
There are two approaches to treatment of migraine headache with BoNT. One is the so-called migraine protocol and the other has been called the “follow-the-pain” protocol. In practice, a third approach is often utilized, which is a combination of the two protocols. No uniform approach has been determined for either protocol, and the studies done to establish doses have been few and preliminary at best. Therefore, there is no established best practice based on acceptable medical evidence in terms of dosing or sites to be injected. What is recommended here is based on a combination what has been published and on the author’s experience as to what has generally worked best. However, the recommendations in this chapter include the protocol in the largest trial to date that showed efficacy of onabotulinumtoxinA in the management of chronic migraine headache [33].
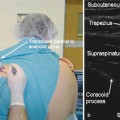
The “migraine protocol” involves injecting BoNT into the corrugator muscles, the procerus muscles, and the temporalis muscles (Fig. 35.1). Many physicians who treat migraine with BoNT include the frontalis muscle as well. I do not do that, but I combine the migraine protocol with the “follow-the-pain” protocol, instead.
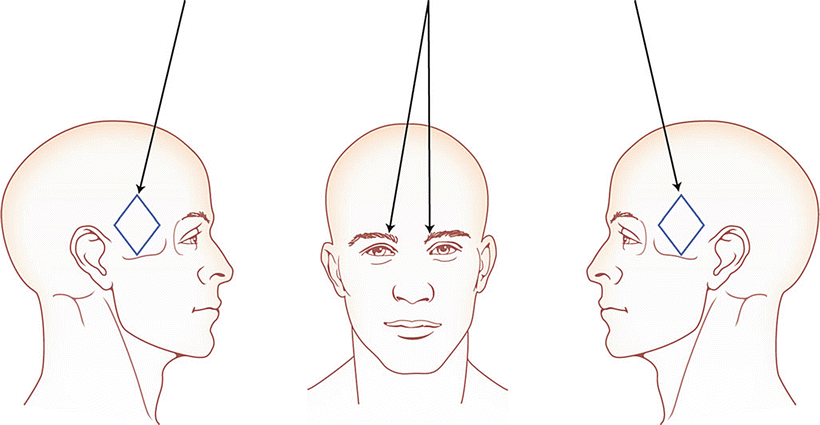

Fig. 35.1
Procerus: between and inferior to the corrugator muscle
The “follow-the-pain” protocol is best characterized as injecting sites in the neck and shoulders that refer to the places where headache pain is felt in the head. This is essentially treating regions in head, neck, and shoulder muscles that refer pain to various places in the head that are characteristic of that person’s migraine headache. An early test of the concept of “follow the pain” is in the 1981 paper by Tfelt-Hansen et al. [35] in Denmark, in which the authors showed that headache could be decreased or eliminated in large percentage of subjects with migraine by injecting muscles outside of head, that is, muscles in the neck and shoulder, that cause pain. An elegant study that specifically looked at sites in muscle that cause pain to be referred specifically to places of headache complaint showed that injection of these referral sites in head, neck, and shoulder, done on a repeated basis, result in a significant reduction in migraine headache frequency and intensity [36].
The muscles and sites to be injected are those that harbor tight and tender taut bands. The taut bands are hardened regions of muscle that are tender to palpation. They are relevant to the patient’s pain when they result in pain referred to the region of headache. This applies not only to migraine but also to tension-type headache and to cervicogenic headache. It also applies to involvement of masticatory muscles that not only directly cause headache pain but that indirectly affect headache by activating trigger points in the cervical musculature.
Chronic Tension-Type and Cervicogenic Headache (CTTH) (Table 35.4)
The headaches of interest in this headache subgroup are those of ≥15 headache days per month, the generally accepted headache frequency for chronic daily headache. There have been few studies of the effect of BoNT on headache that target this group specifically. Many of the studies of chronic daily headache did not distinguish between migraine headache and tension-type headache. Of the few studies that specifically targeted CTTH, three used fixed-dose and fixed-site injection of BoNT-A. They showed no significant reduction in headache frequency [37–39], although in one study, the BoNT-A-treated subjects showed a significantly greater percentage with ≥50 % reduction in headache days compared to the placebo group [39]. One pilot study [40] of great interest looked at the myofascial trigger points (MTrP) that referred pain to typical headache sites, a strategy that builds on many recent studies of the relationship of myofascial trigger points to headache symptomatology, a subject recently summarized in the text by Fernandez-de-las-Peñas et al. [41]. Harden et al. [40] identified trigger points in the neck and upper shoulder, including the sternocleidomastoid and the upper trapezius muscles, and the splenius capitis muscle. They injected 25 units of BoNT-A into each of up to 4 MTrP per subject, using up to 100 units. This was a randomized, double-blinded, placebo-controlled study, but it was quite small, with only 12 subjects in the treated group and 11 subjects in the placebo group. There was a significant decrease in headache days in the BoNT-A-treated group through week 10, but the effect was gone by week 12. This is consistent with the expected duration of effect of BoNT-A on muscle. An open-label extension of the study included 12 subjects from both the BoNT-A and placebo groups. It again showed a significant reduction in headache frequency compared to the original placebo group, for the first 2 months. This study was limited by the small number of subjects and by the small selection of the muscles considered (there were no facial muscles like the masseter and temporalis muscles, and neither the splenius cervicis, oblique capitis inferior, nor semispinalis muscles were injected, although the authors implied that injection of the splenius capitis might have treated multiple underlying muscles). A randomized, single-blinded, placebo-controlled study utilizing a combination of a fixed injection site and “follow-the-pain” approaches also showed significant improvement in the subjects treated with BoNT-A at 1 month, in headache days per month, headache severity, and the in the Henry Ford Headache Disability Inventory [42]. In both the Harden et al. study [40] and the Hamdy et al. study [42], side effects were minor and transient. Another small study of headache associated with myofascial trigger points randomized 45 subjects to treatment with dry needling, lidocaine injections, and botulinum toxin [43




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree