Brain Tumors
Teresa Chapman, MD
LEARNING OBJECTIVES
1. Generate appropriate differential considerations for aggressive intracranial masses in the cerebral hemispheres and in the posterior fossa.
2. Apply the quantification of diffusion restriction to the assessment of a brain tumor’s cellular behavior.
3. Appropriately recommend further imaging with spine MR in the diagnosis of a new brain tumor.
4. Provide a short differential diagnosis for a mixed cystic and solid suprasellar tumor in a pediatric patient.
5. List two tumor types associated with seizures and two tumor types associated with endocrinopathies.
INTRODUCTION
Primary central nervous system tumors are the most common solid pediatric cancer diagnoses.1 A radiologist encountering a new brain tumor diagnosis should attempt to establish extent of disease, declare any emergent complications such as acute hydrocephalus, and attempt to identify the likely tissue types based on the imaging findings and patient age. Understanding the likelihood of aggressive tumor behavior will guide conversations with the referring physicians and with the family. While generating a differential diagnosis for a brain tumor is important, tissue sampling to assess tumor histology, measures of proliferative activity, and molecular evaluations will ascertain the diagnosis and guide subsequent therapy. The World Health Organization (WHO) classifies tumors based on their natural history following surgical resection alone and is part of a standard grading system.2 Lowergrade tumors (WHO grades I and II) are more indolent and are often observed over time without therapy, whereas high-grade tumors (WHO grades III and IV) require intensive therapies beyond surgical resection to control the disease.2 In this chapter, we provide an overview of essential pediatric brain tumors and familiarize the reader with interpretation tools to narrow the differential diagnosis.
IMAGING MODALITIES
Masses in the brain, whether neoplastic or otherwise, may be suspected because of headache, nausea, vomiting, behavioral changes and irritability, or focal neurologic deficits. Initial imaging may be appropriately performed with CT or MRI, depending on the availability and consideration of the ionizing radiation exposure with CT and potential need for anesthesia during MRI.3 Regardless of modality, general features to search for include (1) tumor location; (2) tumor size and infiltration of involved intracranial structures; (3) mass effect including midline shift, obstructive hydrocephalus, and brain herniation; (4) amount of surrounding vasogenic edema, typically seen with rapid growth or parenchymal infiltration; (5) tumor heterogeneity due to intratumoral cysts, hemorrhage, or calcification; (6) contrast enhancement pattern; (7) evidence of hypercellularity based on either hyperdensity on CT or restricted diffusion on MRI; and (8) spread of disease throughout the cerebrospinal fluid (CSF) spaces. Of these diagnostic imaging features, the most concerning signs of an aggressive tumor type include evidence of hypercellularity and CSF spread of disease. Although there are exceptions, large tumors that are predominantly solid and have extensive surrounding vasogenic edema and mass effect tend to be higher in grade.4, 5, 6 and 7 To facilitate the diagnostic process, these three factors help to categorize tumors: patient age, tumor location, and diagnostic imaging features suggesting high grade.
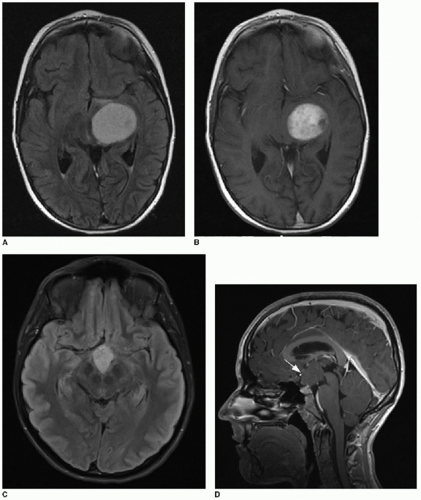
The use of diffusion-weighted imaging in the assessment of a brain tumor’s degree of hypercellularity is helpful and straightforward.4, 5, 6 and 7 To most accurately determine if intratumoral solid tissue is hypercellular, choose a solid area of the mass that enhances and that subjectively shows restricted diffusion based on dark signal on the apparent diffusion coefficiency (ADC) map. As an internal subjective reference to ascertain what tissue might have restricted diffusion, the normal cerebellum has a relatively low diffusion coefficiency and can serve as a visual comparison. An elliptical region of interest within the chosen solid tumor component on the ADC map will provide a calculated coefficiency (Fig. 34.1). The coefficient unit depends on the MR scanner manufacturer. For instance, a coefficient may be presented as 900 mm2/s or 0.9 × 103 mm2/s. As a general rule, hypercellular tumors will have a coefficiency less than 900 mm2/s, whereas less cellular tumors have a coefficiency above 1,400 mm2/s.7,8
Identification of a new tumor, particularly one that is not clearly low grade, merits further evaluation with total spine imaging to assess for drop metastases into the spinal CSF spaces. Advanced neuroimaging with MR spectroscopy and MR perfusion may be
considered in addition to standard sequences.4,9 MR spectroscopy of tumors typically shows decreased levels of N-acetylaspartate and increased levels of choline, with increased levels of lipids and lactate in more rapidly growing tumors.10 Perfusion MRI can distinguish low-grade from high-grade tumors: high-grade tumors tend to have increased angiogenesis with leaky capillaries and therefore elevated relative cerebral blood volume compared with low-grade tumors.9 These techniques, in addition to positron emission tomography (PET), can be particularly useful when attempting to discern recurrent tumor from postradiation gliosis in follow-up examinations.11, 12 and 13
considered in addition to standard sequences.4,9 MR spectroscopy of tumors typically shows decreased levels of N-acetylaspartate and increased levels of choline, with increased levels of lipids and lactate in more rapidly growing tumors.10 Perfusion MRI can distinguish low-grade from high-grade tumors: high-grade tumors tend to have increased angiogenesis with leaky capillaries and therefore elevated relative cerebral blood volume compared with low-grade tumors.9 These techniques, in addition to positron emission tomography (PET), can be particularly useful when attempting to discern recurrent tumor from postradiation gliosis in follow-up examinations.11, 12 and 13
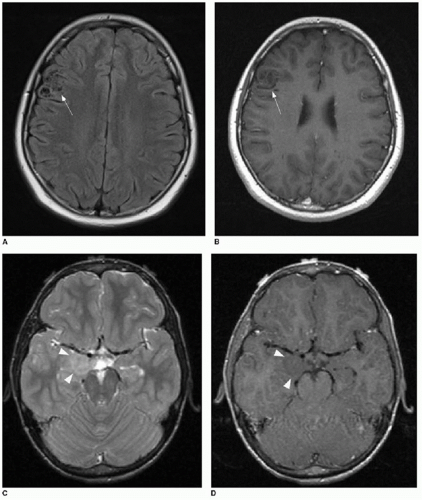
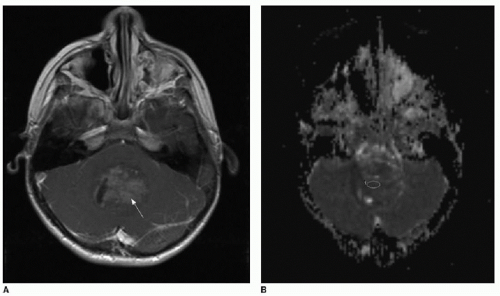 FIG. 34.1 • Demonstration of measuring diffusion coefficiency on ADC map. Axial (A) T1-weighted postcontrast image and apparent diffusion coefficiency (ADC) map (B) from brain MRI performed on a 5-year-old child show a heterogeneously enhancing posterior fossa mass in the midline (arrow, A), pathologically proven to be medulloblastoma. Subjectively, patchy areas within the tumor show dark signal on ADC map, consistent with hypercellularity. Quantification of diffusion is performed by placing an elliptical region of interest (denoted by annotation “1” in image B) over enhancing portion of tumor. Diffusion coefficient here measures 862 mm2/s, consistent with hypercellularity typical of medulloblastoma. |
Specific intracranial tumors are described in this chapter, organized by anatomic location and tumor grade.
SUPRATENTORIAL TUMORS—LOW GRADE, INTRA-AXIAL
Low-grade glioma or astrocytoma can present at any age and is one of the most commonly diagnosed low-grade tumors.14, 15 and 16 Optic tract gliomas, discussed in Chapter 32, may occur in the context of neurofibromatosis type 1 or may be isolated. Although this tumor type favors the optic pathways and the hypothalamus when found in the supratentorial brain, low-grade gliomas can arise anywhere in the central nervous system, including the cerebrum, thalamus, basal ganglia, brainstem, and spinal cord.14,15 Histopathology of low-grade astrocytomas varies and is not associated with any particular anatomic location. These tumors may be histologically described as pilocytic, pilomyxoid, fibrillary, or oligoastrocytoma.14,17 Cellular anaplasia, a histologic marker of a more aggressive growth pattern, is more common when the tumor is more solid than cystic. Therefore, the pilocytic astrocytoma falls within both low-grade and high-grade categories.
On imaging, a low-grade astrocytoma is most commonly characterized by a discrete area of hypodensity on head CT or hyperintensity on T2-weighted sequences on brain MR, without associated mass effect or surrounding vasogenic edema (Fig. 34.2). Enhancement is commonly absent or very minimal. The main diagnostic consideration, when focal, is gliosis. Diffuse astrocytomas tend to have a higher WHO grade.2
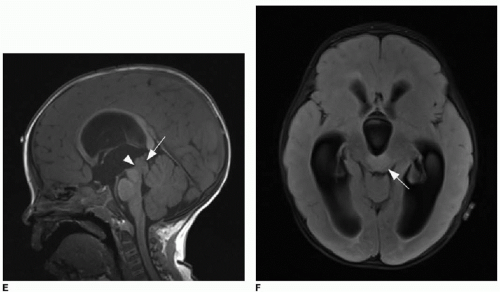
Pleomorphic xanthoastrocytoma (PXA) typically presents in children and young adults. Histologically, this tumor contains a wide variety of cell types, hence the term “pleomorphic.” Importantly, the imaging characteristics of these tumors can also be quite variable.18 Although the periphery of the temporal lobe is reported as the most common site, the tumor can appear in any cerebral lobe and is only very rarely found below the tentorium.18,19 This tumor is characterized classically by a large cyst and a solid peripheral enhancing nodule (Fig. 34.3), although the tumor may contain numerous small cysts rather than a single dominant cyst. Scalloping of the inner calvarial table is a key feature of the PXA, reflecting its slow growth.18 The PXA arises from pial astrocytes, and therefore, a tail of meningeal enhancement might be apparent. The main tumor types in the differential diagnosis of a PXA include pilocytic astrocytoma and ganglioglioma. However, internal solid components may show restricted diffusion,18 generating confusing overlap with highgrade tumors discussed later in this chapter. Anaplastic variants of PXA have a poorer prognosis.20
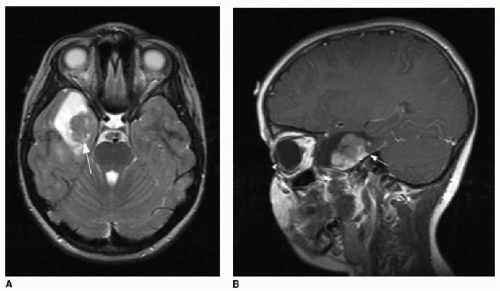
Ganglioglioma also typically presents in children and young adults and is the most common neoplastic cause of focal epilepsy.21, 22 and 23 Other clinical presentations of this tumor include headache or focal neurologic deficit.21,22 As with the PXA, the
ganglioglioma is most commonly found in the temporal lobes, but may arise anywhere.21,22 Histopathology of this tumor is characterized by a mixture of abnormal astrocytic and neuronal components.22 Because of its slow growth, bony remodeling may be evident along its outer margins. The classic imaging features of a ganglioglioma include cystic components as well as solid enhancing tissue.15 The enhancement pattern is characteristically curvilinear (Fig. 34.4).
ganglioglioma is most commonly found in the temporal lobes, but may arise anywhere.21,22 Histopathology of this tumor is characterized by a mixture of abnormal astrocytic and neuronal components.22 Because of its slow growth, bony remodeling may be evident along its outer margins. The classic imaging features of a ganglioglioma include cystic components as well as solid enhancing tissue.15 The enhancement pattern is characteristically curvilinear (Fig. 34.4).
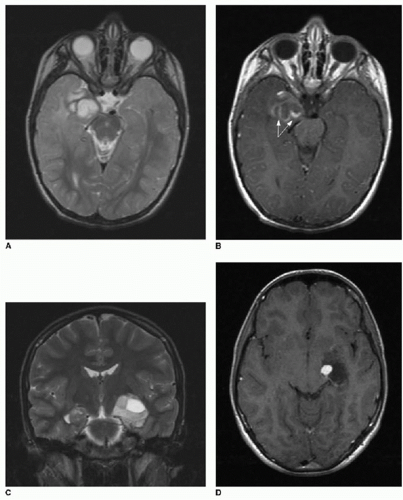
Desmoplastic infantile ganglioglioma (DIG) is a variant of ganglioglioma that usually presents in children under 2 years of age, but has been identified in older children.24, 25 and 26 Common clinical presentations of this tumor include seizures and/or enlarging head circumference.26, 27 and 28 Although the tumor can be quite large at presentation, it is a WHO grade I tumor. Malignant transformation into higher-grade astrocytomas has been described.28,29 Like the ganglioglioma, the DIG demonstrates cyst formation and peripheral nodular enhancement that may be irregular27 (Fig. 34.5).
Dysembryoplastic neuroepithelial tumor (DNET) most commonly presents with seizures in adolescents and young adults. This tumor favors the temporal and frontal lobes and is characterized by a superficial location (typically cortically based) and lack of enhancement and may have multiple small cysts or a dysplastic, nodular appearance (Fig. 34.6).30, 31 and 32 Because it grows slowly, there is usually no adjacent vasogenic edema. However, atypical imaging characteristics, including enhancement, adjacent edema, and multifocality, as well as atypical behavior such as recurrence following surgery, have been described.33 Seizure-free prognosis depends on complete surgical resection and absence of cortical/subcortical injury at the surgical resection site.34
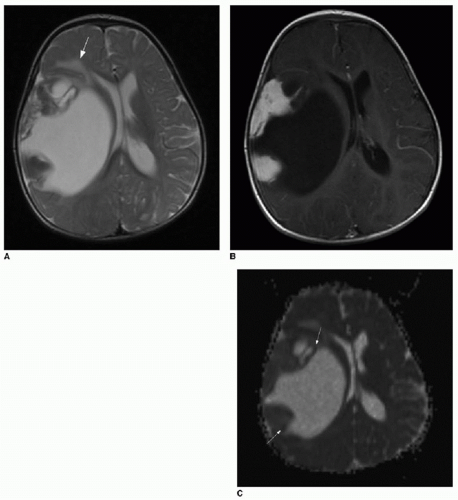 FIG. 34.5 • Desmoplastic infantile ganglioglioma (DIG). Axial (A) T2-weighted and T1-weighted (B) postcontrast brain MR images of 7-month-old infant presenting for enlarging head circumference show a large right cerebral cystic mass with peripheral nodular enhancing components. Mass effect leads to midline shift and right lateral ventricular effacement. Some vasogenic edema is seen at the medial margin of the tumor (arrow in A). C: Apparent diffusion coefficiency map shows patchy areas of dark signal (arrows), subjectively implying areas of hypercellularity. Quantified average coefficient measures 1150 mm2/s here. In spite of the large size, vasogenic edema, and evidence of restricted diffusion, pathology was consistent with a WHO grade II tumor. |
SUPRATENTORIAL TUMORS—HIGH GRADE, INTRA-AXIAL
Primitive neuroectodermal tumor (PNET) is an aggressive, highgrade tumor (WHO grade IV) that arises from small round cells with minimal cytoplasm.35 The supratentorial PNET is rare, and this tumor type more commonly favors the infratentorial brain.36




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree