Vascular and Traumatic Brain Injury
Ramesh S. Iyer, MD
LEARNING OBJECTIVES
1. Identify germinal matrix hemorrhage on cranial ultrasound.
2. List the grading system for germinal matrix hemorrhage.
3. Recognize periventricular leukomalacia in the acute phase on ultrasound, and chronic findings on MR.
4. Describe the typical distributions for both central and peripheral patterns of hypoxic-ischemic encephalopathy.
5. Identify acute arterial ischemic stroke on noncontrast CT and MR.
6. Identify sinovenous thrombosis on noncontrast and contrast-enhanced CT and MR.
7. Describe at least two features on a noncontrast head CT that would be suggestive of nonaccidental trauma.
NEONATAL BRAIN INJURY
Introduction
Neonatal brain injuries may result in death or permanent neurologic impairment. Preterm infants are particularly susceptible because many of the normal compensatory mechanisms for such insults mature during the third trimester. Neonatal brain injuries occur when there is reduced oxygen and nutrient delivery in the setting of hypoxia and ischemia. Glucose stores are depleted, and the brain converts to anaerobic metabolism. The damage is accelerated by excitatory amino acids and oxygen free radicals, with resultant necrosis and apoptosis.1
Cranial ultrasound is often the initial imaging modality, particularly in preterm or critically ill infants. The anterior fontanelle is used as the acoustic window, and sagittal and coronal images of the brain are acquired. Ventricular size and the presence of intraventricular hemorrhage or other extra-axial collections are readily evaluated with US at the patient’s bedside. However, small or subtle parenchymal abnormalities may be difficult to detect. MR offers excellent sensitivity and specificity for characterizing neonatal brain morphology and patterns of injury. It is important to remember that neonatal brains have higher water content than do those of older children because most structures have yet to be myelinated. CT is typically used only for select circumstances with neonates, such as characterizing intracranial hemorrhage or preoperative planning for ventricular shunting.
Germinal matrix hemorrhage
The germinal matrix is a highly vascular gelatinous substance located in the walls of the ventricles and subventricular zones. It is a transient area of neuronal and glial cell proliferation that matures in utero and involutes by 35 weeks’ gestational age. The last area to involute is in the caudothalamic groove, located between the head of the caudate nucleus and the thalamus. The vessels of the germinal matrix are thin walled and fragile, making it prone to hemorrhage.1,2
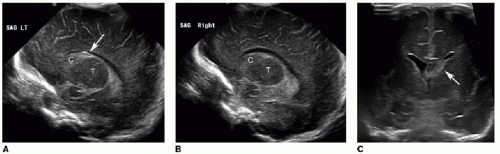
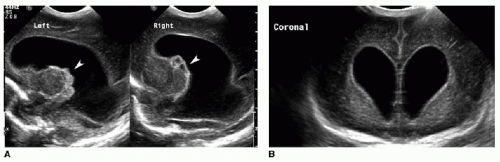
Germinal matrix hemorrhage is classified into four categories (Table 31.1). Grade 1 hemorrhage is confined to the germinal matrix itself, typically within the caudothalamic groove (Fig. 31.1). Grade 2 hemorrhage extends into the ventricles, which remain normal in size. With grade 3 bleeds, the ventricles become dilated (Fig. 31.2). Grade 4 involves parenchymal hemorrhage related to venous infarction and is not necessarily related to the milder grades, nor does it represent extension of intraventricular blood into the adjacent parenchyma (Fig. 31.3).3 Ventricular shunting is performed for higher-grade injuries and hydrocephalus. Grades 3 and 4 germinal matrix hemorrhages generally have higher morbidity and mortality rates.
Table 31.1 CLASSIFICATION OF GERMINAL MATRIX HEMORRHAGE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Periventricular leukomalacia (white matter injury of prematurity)
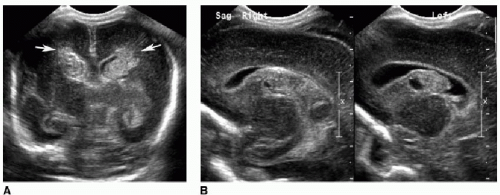
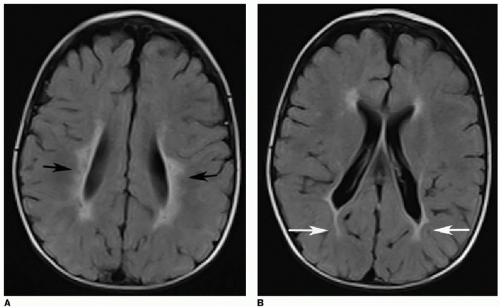
Periventricular leukomalacia, or white matter injury of prematurity, is another type of ischemic insult affecting infants younger than 34 weeks’ gestational age. This occurs because immature oligodendrocytes are more susceptible to direct glutamate toxicity, oxygen free radical effects, and inflammatory cytokines as a result of ischemia.1 As the name suggests, this injury involves the periventricular and deep white matter. Cranial US is often the initial imaging evaluation. Normal neonatal deep white matter is diffusely and homogeneously echogenic, particularly during the first week of life. Acute white matter injury manifests as a focal region of heterogeneous or increased echogenicity, often described as flame shaped (Fig. 31.4). This edema or hemorrhage occurs during the first week of life. MR is typically performed 24 to 72 hours after the suspected injury and will show increased T2-weighted signal, diffusion restriction, and petechial hemorrhages. After 3 to 4 weeks, cystic or cavitary encephalomalacia develops in the periventricular white matter. MR findings of prior white matter injury in older children include high T2/FLAIR signal abutting the ventricles, and white matter volume loss (Fig. 31.5).1,2,4, 5 and 6
Hypoxic-ischemic encephalopathy in term infants
Hypoxic-ischemic encephalopathy (HIE), or birth asphyxia, occurs in about 0.5% of live births in the United States and is among the most important causes of cerebral palsy and other severe neurologic disability in children.2,7 HIE in the term infant
is highly variable and depends on the severity and duration of the ischemic insult. MR is the modality of choice when HIE is suspected, and is performed 3 to 7 days after the insult for optimum sensitivity and accuracy. Earlier imaging may underestimate the injury because of delayed cell death, such as apoptosis. Diffusion-weighted imaging (DWI) often depicts the extent of cytotoxic edema better than do conventional T1- and T2-weighted sequences.2,8
is highly variable and depends on the severity and duration of the ischemic insult. MR is the modality of choice when HIE is suspected, and is performed 3 to 7 days after the insult for optimum sensitivity and accuracy. Earlier imaging may underestimate the injury because of delayed cell death, such as apoptosis. Diffusion-weighted imaging (DWI) often depicts the extent of cytotoxic edema better than do conventional T1- and T2-weighted sequences.2,8
There are two predominant patterns of HIE. The deep or central pattern of HIE occurs in the setting of profound asphyxia. The affected structures are very metabolically active and therefore most susceptible to interruptions in blood supply. Central HIE affects the ventrolateral thalami, basal ganglia, hippocampi, brainstem, and perirolandic cortex. MR will show elevated T1-weighted signal, variable T2-weighted signal, and restricted diffusion in the deep gray structures (Fig. 31.6). MR spectroscopy demonstrates elevated lactate within the basal ganglia and thalami.2,5,8
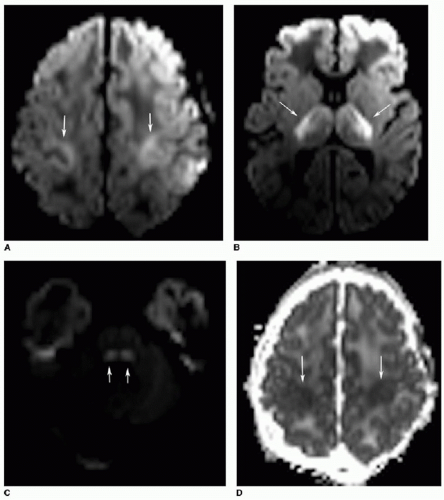
The peripheral pattern of HIE occurs with mild to moderate hypotension. These injuries occur when blood is shunted to
the more vital portions of the brain. Deep gray structures, the brainstem, and cerebellum are spared by milder ischemic insults. Instead, peripheral HIE involves “watershed” zones of the cerebral hemispheres, such as between the middle cerebral and anterior cerebral arterial circulations (Fig. 31.7). Similar to the central pattern, restricted diffusion is noted during the first week after the insult, followed by T2-weighted hyperintense gliosis and atrophy over time.2



the more vital portions of the brain. Deep gray structures, the brainstem, and cerebellum are spared by milder ischemic insults. Instead, peripheral HIE involves “watershed” zones of the cerebral hemispheres, such as between the middle cerebral and anterior cerebral arterial circulations (Fig. 31.7). Similar to the central pattern, restricted diffusion is noted during the first week after the insult, followed by T2-weighted hyperintense gliosis and atrophy over time.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree