Chapter 26 Breast cancer
Anatomy
Lymphatic drainage
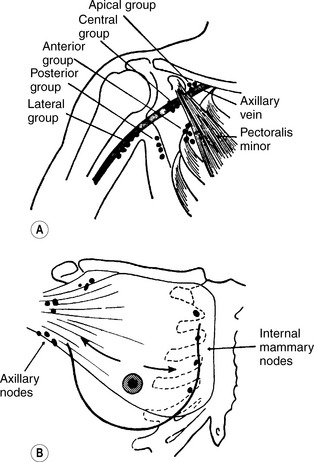
The principal lymphatic drainage of the breast (Figure 26.1) is to the axillary nodes lying between the second and third intercostal spaces. Additional drainage occurs to the supraclavicular nodes through the pectoralis major and to the internal mammary chain adjacent to the sternum.
Pathology
Aetiology
Genetic factors
High-risk mutations
• three first- or second-degree relatives with breast or ovarian cancer
• one first-degree relative with bilateral breast cancer
• two first- or second-degree relatives with breast cancer diagnosed under the age of 60 or ovarian cancer at any age on the same side of the family.
Histology
A lump in the breast may be benign or malignant. Benign lesions include cysts, fibroadenomas and papillomas. Malignant tumours mainly arise from the glandular epithelium (adenocarcinomas). Breast cancers are classified as of no special type or of special type. The majority (80%) are of no special type. The histological types of breast cancer are shown in Table 26.1.
Table 26.1 Classification of invasive breast cancer
| 1. | No special type |
| 2. | Special type |
| a. Tubular | |
| b. Mucoid | |
| c. Cribriform | |
| d. Papillary | |
| e. Medullary | |
| f. Classic lobular |
Diagnosis
Mammography
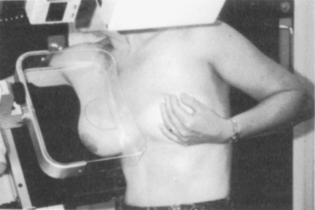
Breast screening by mammography (Figure 26.2) in the UK is recommended to women between the ages of 50 and 69 every 3 years. In the UK, two views of the breast are obtained. In the countries where it has been widely applied (e.g. in Sweden), its use has been shown to reduce the mortality of breast cancer by 30%.
Features suggestive of malignancy are small microcalcifications, stellate opacities with ‘legs’ extending into the surrounding tissues (Figures 26.3 and 26.4) or distortion of architecture. Mammography may also show enlarged nodes in the axilla. About 15% of cancers are not detected by mammography and nearly 4% are neither palpable nor visible on mammography.
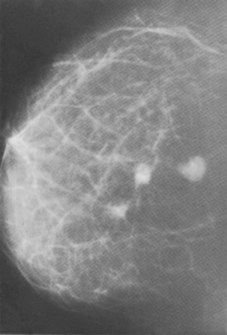
Figure 26.3 Mammogram showing three spiculated tumours in the breast.
(Courtesy of Dr R. Peck, Sheffield)
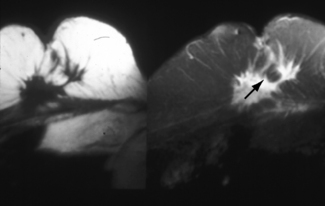
Figure 26.4 MRI scan showing recurrent cancer in the breast.
(Courtesy of Mr M. Dixon, Edinburgh Breast Unit)
MRI scanning (Figure 26.4) may have an important role in the assessment of (a) the local extent of the primary tumour and of multifocality in younger women where the density of the breast is often a limiting factor to the resolution of mammography and (b) recurrent disease in the irradiated breast 18 months or more after radiotherapy has been completed. MRI may show 10–30% additional invasive cancer in patients presenting with a unifocal lesion. However, MRI may lead to the overestimate of the size of lesions, potentially resulting in unnecessary mastectomies.
Breast ultrasound
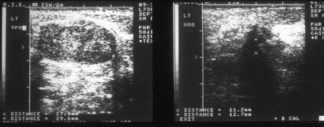
Ultrasound of the breast has an important role in helping to distinguish benign from malignant lesions, particularly when mammography is normal or equivocal. Malignant tumours tend to show an abnormal echo pattern (Figure 26.5). Colour Doppler ultrasound may show changes caused by increased tumour vasculature both in primary tumours and in lymph nodes.
Staging
Staging is important to assess the local, regional and metastatic spread of breast cancer since management may differ significantly depending on the extent of the disease. Staging involves clinical, radiological and laboratory assessment. The simplest staging system which is still in use is shown in Table 26.2. However, the TNM classification of the International Union Against Cancer (Table 26.3) has gained widespread acceptance.
Table 26.2 Clinical staging of breast cancer
| Stage | Clinical findings |
|---|---|
| I | Freely movable (on underlying muscle). No suspicious nodes |
| II | As stage I but mobile axillary node(s) on the same side |
| III | Primary more extensive than stage I, e.g. skin invaded wide of the primary mass or fixation to muscle. Axillary nodes, if present, are fixed; or supraclavicular nodes involved |
| IV | Extension beyond the ipsilateral chest wall area, e.g. opposite breast or axilla; or distant metastases |
Table 26.3 TNM classification of breast cancer
| Stage | Clinical findings |
|---|---|
| Primary tumour | |
| Tis | Carcinoma-in-situ |
| T0 | No demonstrable tumour in the breast |
| T1 | Tumour less than 2 cm in greatest dimension confined to the breast |
| T1a | Tumour 0.5 cm or less in maximum dimension |
| T1b | Tumour more than 0.5 cm but not more than 1 cm in greatest dimension |
| T1c | Tumour more than 1 cm but not more than 2 cm in greatest dimension |
| T2 | Tumour more than 2 cm but less than 5 cm in greatest dimension |
| T3 | Tumour more than 5 cm in its greatest dimension |
| T4 | Tumour of any size with direct extension to chest wall or skin |
| T4a | Fixation to chest wall |
| T4b | Oedema, infiltration or ulceration of the skin of the breast |
| T4c | Both of above |
| Regional lymph nodes | |
|---|---|
| N0 | No palpable nodes |
| N1 | Mobile ipsilateral nodes |
| N2 | Fixed ipsilateral nodes, fixed to each other or to other structures |
| N3 | Ipsilateral internal mammary nodes |
| Distant metastases | |
|---|---|
| M0 | No distant metastases |
| M1 | Distant metastases including skin involvement beyond the breast area and supraclavicular nodes |
Treatment of ductal carcinoma-in-situ
Prognostic factors
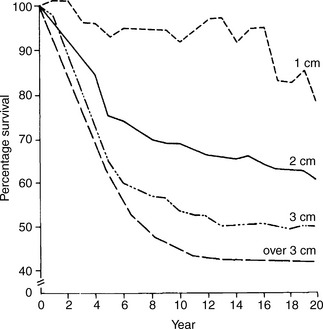
While there are a large number of biological prognostic factors for breast cancer, none has surpassed the value of assessing the number of histologically involved nodes and tumour size. There is a direct correlation between number of involved nodes and survival (Figure 26.8). Ten-year survival is about 40–65% with one to three positive nodes, and 20–42% for those with 10 or more positive nodes. Ten-year survival is about 65–70% in women with negative nodes. By contrast, in excess of 50% of all women who are axillary node positive die within 10 years despite treatment.
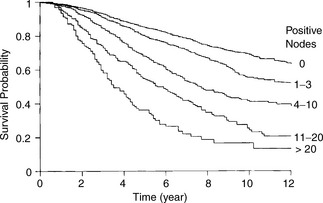
Figure 26.8 Survival by axillary node status.
(Reproduced with permission from Veronesi U (ed.) Baillière’s Clinical Oncology, 1988)
Within any category of nodal status, tumour size is an independent prognostic factor. The decline in survival with increasing size of the primary tumour is shown in Figure 26.9. Less than 30% of patients with stage IIIb disease (T4) are alive at 10 years. Similarly, survival declines with increasing tumour grade. Five-year survival falls from 80% for grade 1 to 25% for grade 3.
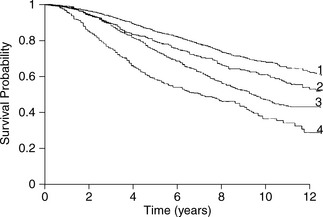
Oestrogen receptor status is predictive for disease-free and overall survival. Irrespective of stage, ER positivity predicts for longer disease-free (Figure 26.10) and overall survival. Higher recurrence and lower survival rates are found in ER-negative patients. About 60% of ER-positive patients will respond to hormonal manipulation. Progesterone receptor (PgR) status may also help. Oestrogen stimulates PgR production in normal reproductive tissue and in human breast cancer cell lines. The highest response and disease-free survival rate is seen in ER+/PgR+ tumours. Very few tumours are ER−/PgR+, consistent with the production of progesterone receptors being dependent on oestrogen synthesis. Lowest response and disease-free survival rates are seen in ER−/PgR− tumours (Figure 26.11).
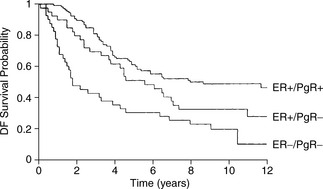
Figure 26.11 Disease-free survival by oestrogen and progesterone receptor status in pathological stage II patients.
(Reproduced with permission from Baillière’s Clinical Oncology, International Practice and Research, 1988)
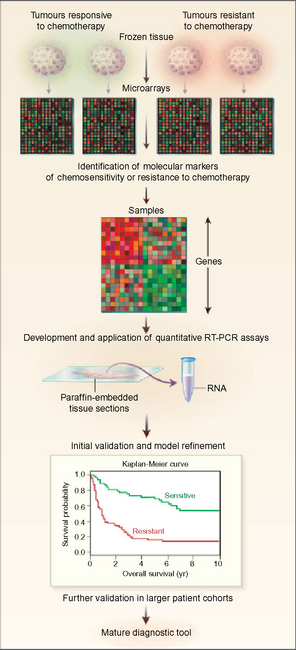
Gene profiling
More recently, microarray based gene profiling has enabled thousands of genes to be studied in a one tumour. A microarray consists of known and unknown DNA samples on a solid slide (Figure 26.12). The probes may be cDNAs or oligonucleotides of varying length. The sequence hybridized to probes on the array can be fluorescently labelled. Expression signatures based on the expression level of large nunbers of genes can be determined reflecting the properties of the cells studied. There is now preliminary evidence that DNA microarrays may be able to discriminate patients at higher or lower risk of systemic relapse among conventionally ‘low risk’ node-negative patients treated by breast conserving therapy. These findings will have to be validated prospectively in larger data sets before they can be used to determine management for individual patients.
Treatment of early breast cancer
Mastectomy or breast conservation
Conservation therapy (limited surgery and postoperative radiotherapy)
Alternatively, a more extensive local excision of the whole quadrant affected in the breast (quadrantectomy) may be carried out. The advantage of quadrantectomy is that local recurrence rates (about 1% at 5 years when combined with postoperative radiotherapy) are lower than after wide local excision and radiotherapy (about 5% at 5 years). The disadvantage is that the cosmetic result is generally poorer because of the asymmetry caused by the greater volume of tissue removed. Quadrantectomy or a very wide local excision may be considered in patients refusing or not fit for mastectomy or breast radiotherapy. This may be advisable in some older patients where co-morbidity may compromise suitability for mastectomy. Criteria for breast conservation are summarized in Table 26.4.
Table 26.4 Criteria for breast-conserving therapy
1. Tumours up to 3 cm 2. Satisfactory cosmetic result anticipated 3. Postoperative whole breast radiotherapy technically feasible 4. Medically fit for surgery 5. Clear histological margins at primary excision or re-excision 6. Able to attend for regular clinical and mammographic follow up |
Postoperative radiotherapy
After breast-conserving surgery
In general, postoperative whole-breast irradiation should be delivered following wide local excision or quadrantectomy as part of breast-conserving therapy. A number of randomized trials comparing wide excision alone with appropriate systemic therapy have shown overall a fourfold reduction in local recurrence from the addition of whole breast irradiation (Table 26.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree