, Leonid Tiutin2 and Thomas Schwarz3
(1)
Russian Research Center for Radiology and Surgery, St. Petersburg, Russia
(2)
Department of Radiology and Nuclear Medicine, Russian Research Center for Radiology, St. Petersburg, Russia
(3)
Department of Nuclear Medicine Division of Radiology, Medical University Graz, Graz, Austria
Abstract
Currently breast cancer (BC) is the most frequent of all oncological diseases, both in incidence and in mortality. Suffice it to say that annually more than one million cases of BC are registered in the world. In the USA this disease is detected in roughly 180,000 women per year. In Russia, BC incidence is continually growing. In 1980 it constituted 22.6 per 100,000 of the population, while in 2003 it was already 42.8. The peak of BC incidence falls on the age of 55–59 years (138.9 per 100,000 of the population) and 60–69 years (145.2 per 100,000 of the population) (Semiglazov et al. 2001).
Currently breast cancer (BC) is the most frequent of all oncological diseases, both in incidence and in mortality. Suffice it to say that annually more than one million cases of BC are registered in the world. In the USA this disease is detected in roughly 180,000 women per year. In Russia, BC incidence is continually growing. In 1980 it constituted 22.6 per 100,000 of the population, while in 2003 it was already 42.8. The peak of BC incidence falls on the age of 55–59 years (138.9 per 100,000 of the population) and 60–69 years (145.2 per 100,000 of the population) (Semiglazov et al. 2001).
The classification of breast cancer follows the TNM scoring.
4.1 Diagnosis of Breast Cancer
Self-examination (visual examination and palpation) of the mammary glands, according to WHO recommendations should be done once a month during the first days after menstruation, starting from the age of 25 years.
Physical examination of the mammary gland done by an oncologist is of much importance in diagnosing breast cancer. During the examination, symmetry of location and form of the breast glands, the state of the skin and nipples etc. are assessed. Besides superficial and deep palpation of the breast glands, axillary, subclavicular and cervico-supraclavicular lymph nodes are done. The diagnostic accuracy of this method is largely determined by the specialist’s professional skill, but on the whole it does not exceed 45–60% for primary tumor and 50–70% for metastases to regional lymph nodes (Portnoi et al. 1995).
Currently, the main instrumental method of radiological examination of BC patients is still X-ray mammography. The improvement of X-ray mammography apparatuses has permitted a decrease of radiation exposure to 0.002–0.003 Gy, and in modern low-dose X-RAY MAMMOGRAPHY the effective dose falling on the breast gland has decreased to 0.0002–0.0003 Gy. The analysis of some examination results has showed that use of X-ray mammography as screening method of diagnosing BC leads to decrease in mortality by no less than 30% (Semiglazov et al. 2001).
X-ray mammography sensitivity according to different authors varies from 70% to 92%. At the same time, minimum forms of BC are detected in 76.5% of cases. However, the relatively low specificity of the method remains the main limiting factor of its use. Suffice it to say that in roughly 30% of cases when mammography diagnoses BC, the results of postoperational biopsy attest to the benign nature of the lesion (Tsyb and Tlepshukov 1989; Nystrom et al. 1993; Kharchenko et al. 1996, 1999; Kholin 1996).
Currently, ultrasound (US) is widely used for diagnosing BC; along with X-ray mammography, it can be considered as the main method of screening diagnosis of BC. In particular, ultrasound mammography takes the leading part in differentially diagnosing between solid and cystic masses. The disadvantages of USD include the impossibility of visualizing microfoci of malignization in the presence of diffuse proliferative fibroadenomatosis in the patient. Some authors think that US does not have any independent significance in diagnosing BC since it is characterized by a relatively low sensitivity and may be used only for differential diagnosis of cysts and solid masses and in women younger than 30 years, and for pregnant and nursing women in case of detection of a palpable mass (Basset and Kimme-Smith 1991; Harvey et al. 2008). According to some authors (Zaitsev et al. 1996, 1997), mammography should be used as the first stage of examination in elderly women in case nonpalpable masses are detected, in the presence of tumor focus localized behind the nipple. The authors recommend implementing US mammography in young women, in case of distinctly palpable masses as well as in cases when tumor focus is localized in areas of the breast gland inaccessible to X-ray mammography.
CT is an efficient additional method of diagnosis, permitting significant additional information to be obtained in case of the infiltrative-edematous form of breast cancer, tumor relapses and neoplasms localized in the retromammary space. This method is particularly valuable in the presence of metastases to lymph nodes of axillary and subclavicular areas (Taira et al. 2008).
MRI has a high efficacy when used in the dynamic regime with contrast. This method, when used without paramagnetic contrast agents, is less informative than mammography. According to different authors, the sensitivity of dynamic contrast-enhanced MRI (DCMRI) in diagnosing breast cancer reaches 95–98%, but its specificity remains relatively low (50–80%) and depends to a large extent on the state of surrounding glandular tissue (Belenkov et al. 1996; Arzumanova et al. 1999; Bedrosian et al. 2000; Dewey and Martus 2008). The tangible advantage of DCMRI over X-ray mammography and US is its ability to detect within one examination metastatic lesion of lymph nodes of the retromammary space and anterior mediastinum. In consequence, this method of radiologic examination may be used for assessing the dissemination of the tumor process.
Recently, the question of using radionuclide methods of visualization in diagnosing BC has been widely discussed. 99mTc-technetryle scintigraphy is a highly informative method of diagnosis in detecting primary focus and regional metastases. The exact mechanism of 99mTc-technetryle uptake by tumor cells is not clear for now. It is known that about 90% of this radiopharmaceutical (RP) accumulates in the mitochondria of tumor cells. At the same time, other research has shown that 99mTc-technetryle uptake depends to a larger extent on the activity of cell proliferation than on the density of mitochondria and processes of neovascularization. The sensitivity, specificity and diagnostic accuracy of the method in detecting palpable malignant breast neoplasms according to some authors make up 84%, 80% and 82% respectively (Khalkhali and Vargas 2001; Liang et al. 2008). It should be noted that the method of two-stage scintimammography done in the regime of double scanning (10–20 min and 2 h after the introduction of RP) permits detection not only of the primary focus and metastatic lesions of lymph nodes but also the resistance of the primary focus and regional metastases to chemotherapy since 99mTc-technetryle and tetrophosmin used in this method are substrates of MDR-I gene-encoded P-glycoprotein, ensuring active transport of xenobiotics from cells.
SPECT is also rather widely used in clinical practice, along with planar scintigraphy, and has some advantages over the latter. For example, Nagaraj et al. (1994) detected on the material of 34 BC patients significant information from 99mTc-technetryle SPECT in confronting the data with those of a planar examination done with the same RP. With roughly the same values of sensitivity, SPECT was characterized by a much higher specificity in diagnosing primary tumor node compared with planar scintimammography (70% and 50% respectively). The specificity of the SPECT examination in detecting metastatic lesions of axillary lymph nodes was also higher than that of planar scintimammography (100% and 70% respectively). However, it should be noted that the resolving capacity of modern gamma-cameras does not exceed 1 cm. This restricts the suitability of using scintimammography (both planar and SPECT) in the presence of a primary tumor focus and deposits in lymph nodes with diameter less than 1 cm.
Diagnosis of metastases to regional lymph nodes is still a weak link in assessing generalization of breast cancer. Direct lymphangiography used for assessing regional lymphatic metastasis has not received wide use because of its invasivity and danger of complications. Nuclide lymphoscintigraphy with technetium-based RP is a more available and less dangerous method, but like direct lymphoscintigraphy it does not allow differential diagnosis between hyperplasia, inflammatory lymphadenitis and metastatic lesion of lymph nodes (Palmedo et al. 1996; Ryannel et al. 1998).
4.2 PET in Diagnosis of Breast Cancer
4.2.1 Diagnosis of Primary Tumor Focus
Breast cancer is characterized by high glycolytic activity, which results in intensive 18F-FDG uptake in tumor tissue. The level of glycolysis, and consequently that of 18F-FDG hyperfixation, depends on the histological type of primary tumor focus.
In 1989, Kubota et al. (1989) described focal RP uptake in a BC patient. A pilot study done by Wahl et al. (1991) in 12 patients with histologically confirmed BC revealed the high informative value of 18F-FDG PET. The size of tumor in all the examined patients exceeded 3 cm. Later examinations done in a big patient group (124 patients) confirmed that 18F-FDG PET is a highly informative method of detecting primary tumor focus in BC (Phelps 2004). The sensitivity of the method made up 92% and its specificity was 92%. The size of tumor was smaller than 3 cm in 79 patients. Summary data on the informative value of 18F-FDG PET in detecting primary tumor focus in BC patients are displayed in Table 4.1.
Table 4.1
Sensitivity, specificity and diagnostic accuracy of 18F-FDG PET in diagnosis of the primary tumor in BC patients according to various authors
Author | Year | Patient number | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|
Zhao et al. | 2007 | 24 | 92.7 | 90.1 | 91.4 |
Mavi et al. | 2006 | 152 | 94.1 | 89.3 | 90.2 |
Tyutin et al. | 2003 | 78 | 97.1 | 100 | 97.8 |
Scheidhauer et al. | 2001 | 117 | 93.0 | 75.0 | 89.0 |
Rostom et al. | 93 | 91.0 | 83.0 | 89.0 | |
Noh et al. | 27 | 96.0 | 100 | 97.0 |
For example, Schirrmeister et al. (2001) have detected that in case of infiltrating ductal carcinoma a higher RP uptake by tumor cells is observed than in lobular tumor. Besides, direct correlation between 18F-FDG uptake [in particular, the standardized uptake value (SUV)] and the degree of tumor malignancy has been observed. In most cases, primary tumor is defined as the focus of high RP uptake (Fig. 4.1). SUV values in tumor tissue and T/NT ratio significantly vary, however, according to our data, maximum SUV (SUVmax) averages 3.71 ± 0.21 and T/NT ratio 7.08 ± 0.99 (Granov 2007).
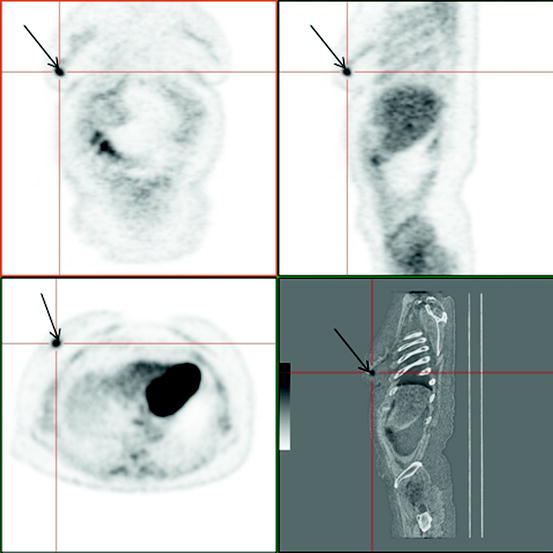

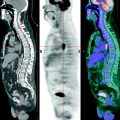
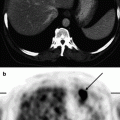
Fig. 4.1
Patient, 35 years old. Breast cancer T3N0M0. 18F-FDG PET / CT: Pathological uptake in the external quadrant of right breast (arrows)
Currently much attention is given in literature to correlation between 18F-FDG uptake by BC cells and the level of expression of the p53 gene. This gene governs the transcription of at least two sets of genes. One of the set is responsible for a temporary stop in cytokinesis in the phase G1 and the other controls apoptosis initiation. Besides, the non-mutant gene p53 suppresses expression of genes responsible for synthesis of the receptors GLUT1 and GLUT4, whose number determines 18F-FDG uptake by tumor cells. According to some authors, there is a strong direct correlation between the level of uptake of the mutated protein p53 (in consequence of mutation of the corresponding gene) in breast tissue and the level of 18F-FDG hyperfixation in the area of primary tumor focus (Hiraki et al. 1988; Utech et al. 1996; Crippa et al. 1998; Rostom et al. 1999).
A number of works are devoted to comparison of the diagnostic accuracy of 18F-FDG PET with that of other methods of radiologic visualization. For example, according to Bassa et al. (1996) the sensitivities of 18F-FDG PET, mammography and USD were, respectively, 100%, 62.5% and 87.5%. The size of primary tumor focus ranged from 1.7 to 9.0 cm. According to Tyutin et al. (2001), the sensitivity, specificity and diagnostic value of 18F-FDG PET in detecting primary tumor in BC patients was 96.8%, 100% and 97.2% respectively. In this work, the sensitivity of 18F-FDG PET was higher for the group of patients with palpable BC masses (Fig. 4.2).
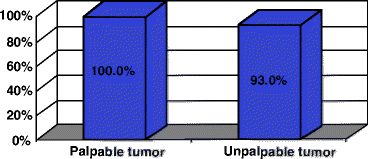

Fig. 4.2
Sensitivity of PET with 18F-FDG depending on size of a primary tumor focus
At the same time, no significant difference in efficacy of the method was observed depending on concomitant fibroadenomatosis (Fig. 4.3). The accuracy of X-ray mammography, US and DCMRI in this work was significantly lower compared with that of 18F-FDG PET and was defined as 87.3%, 90.1% and 90.3% respectively.
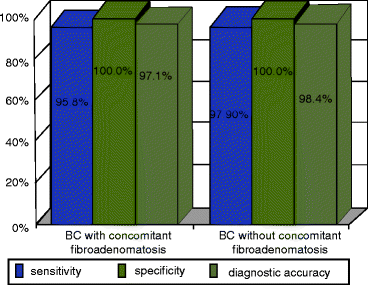

Fig. 4.3
Informativity of PET with 18F-FDG in diagnosis of BC depending on the presence of concomitant fibroadenomatosis
False-negative cases in 18F-FDG PET are usually conditioned by small size of the formation (less than 0.5 cm). Also, Paget’s cancer (tumor of the nipple and breast areola) is characterized by low metabolic activity of glucose and may also be a cause of false-negative results. False-positive data may be obtained in patients after biopsy and are usually conditioned by an inflammation area in this site. Implementing a delayed scan (120–240 min after RP introduction) allows high accuracy in differentially diagnosing between tumor tissue and inflammatory changes. Noh et al. (1998) revealed that in BC an increase in SUV in tumor (at least by 10%) was observed. At the same time this index does not significantly change in inflammatory areas after the repeated scan or its further decrease is observed. Similar data were obtained by Boerner et al. (1999) who examined 29 BC patients. Data collection was done 1.5 and 3 h after RP introduction. A significant increase in the tumor/background ratio was observed during the second scan (from 1.2 ± 0.6 to 6.1 ± 3.0).Tumor detectability according to the authors increased from 83% to 93%.
In some studies, the patient’s position during scanning (on their back or on their stomach) influences the efficacy of the method. SUV and T/NT ratio values in most cases were higher when examining patients lying on their stomachs. However, these data had no impact on the sensitivity, specificity and diagnostic accuracy of PET (Greco et al. 2001).
So it is clear that PET is an efficient additional method of diagnosing primary tumor focus in BC. However, its use is justified only in difficult diagnostic situations. Examining patients suspected of BC should always begin with physical examination (palpation) of the breast glands and regional lymph nodes (axillary and subclavicular). If pathologic changes are detected, recourse is made to X-ray mammography and USD as well as to USD-controlled puncture biopsy. In difficult clinical situations as well as for the purpose of detecting metastases to internal lymph nodes, it is suitable to use DCMRI, whose results may be completed by data from 99mTc-technetryle scintimammography. If after this examination there remain doubts as to the malignancy of the pathologic process, 18F-FDG PET should be implemented. Trepan biopsy is done at any stage of diagnostic search if signs of malignant neoplasm are discovered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree