, Valery Kornienko2 and Igor Pronin2
(1)
N.N. Blockhin Russian Cancer Research Center, Moscow, Russia
(2)
N.N. Burdenko National Scientific and Practical Center for Neurosurgery, Moscow, Russia
Breast cancer (BC) is the most common malignancy in women and occupies the first place in the structure of morbidity and mortality from malignant neoplasms in the female population of Russia: the absolute number of cases and deaths in 2013 has exceeded 57,000 and 25,000, respectively. According to Davydov and Axel (2014a, b) the incidence of malignant breast tumors tends to increase: from 42.8 per 100,000 in 2007 to 46.1 in 2012 among the female population of the Russian Federation. The growth rate was 7.7%, while mortality from malignant breast tumors tended to decrease from 17.1% in 2007 to 15.9% in 2012.
The risk of developing breast cancer increases with age: in 0.8% of cases, the disease manifests before the age of 30 years and in 6.5% at the age of 30–40 years, and more than 90% of breast cancer cases occur after 40 years of age.
The most common histological type of breast cancer is invasive ductal carcinoma, and lobular cancer is the second most common histological type of invasive breast cancer (Althuis et al. 2004). Less common are mucinous forms, tubular and cribriform cancer, and papillary, adenoid cystic, and secreting histological types of breast cancer characterized by low rates of metastasizing to the regional lymph nodes and a more favorable prognosis.
Breast cancer ranks second after lung cancer by frequency of brain metastases (Baker 1942; Baker et al. 1951; Lang and Slater 1964; Takakura et al. 1982; Lin et al. 2004; Stelzer 2013). According to several authors, MTS in the brain develops in 10–35% of all cases of breast cancer (Cifuentes and Pickren 1979; Le Chevalier et al. 1985; Lassman and DeAngelis 2003; Lin et al. 2004; Leone and Leone 2015) and 10% of patients in disseminated breast cancer (Boogerd et al. 1993; Fisher et al. 1997). According to our data, metastases of breast cancer accounted for 23% of cases.
Intracranial metastases of breast cancer are more common in case of massive extracranial metastasizing in 54% (Stark 2011). While lung cancer brain metastases are more common in men, in women breast cancer, metastases prevail. The incidence of breast cancer in men and women is estimated to be 1:100. Breast cancer metastases to the brain typically occur between 2 and 3 years from the time of diagnosis of the primary disease (van Eck et al. 1965; Nussbaum et al. 1996). According to Grigorov and Plotnikova (2012), the time interval from the detection of the primary tumor to the appearance of cerebral metastases depends on the initial stage of the disease and treatment of the primary lesion and, according to the authors, is 1 (at stage IV) to 4 years and 1 month (at stage IIA).
According to some authors, breast cancer metastases manifest in the brain as a single neoplasm (Zedeler et al. 1992; Flowers and Levin 1993; Yamada et al. 1997), according to other sources, these are often multiple lesions (Altundag et al. 2007).
In metastatic breast cancer, chemotherapy is effective in 70–80% of cases, while patients with treatment failure die from distant metastases. The average life expectancy in patients with stage IV breast cancer is 18–24 months, and this figure varies depending on the site of metastases. The 5-year survival rate of patients with breast cancer with distant metastases is 19%, with the disease having the worst prognosis in cases of visceral metastases (Zimm et al. 1981). However, Tevaarwerk et al. (2013) point out the apparent improvement in survival rates among patients with breast cancer over the last 30 years.
Breast cancer metastasizes to the cerebral hemispheres, cerebellum, brainstem, and spinal cord in 80–85%, 10–15%, 3–5%, and less than 1% of cases, respectively. Breast cancer metastases to the brain can spread through the bloodstream and lymphatic system. The presence of a lymphogenic metastasis determines an unfavorable outcome (Semiglazov et al. 2006).
In 5 to 20%, the meninges are involved (Grossman and Krabak 1999; Altundag et al. 2007; Kim et al. 2012; Scott and Kesari 2013), while in a percentage of cases, metastases in the spinal cord are detected in addition to brain lesions. According to Scott et al. (2016), the survival of patients with breast cancer leptomeningeal metastases is on average 4 months.
Given the frequent manifestation of breast cancer metastases in the form of cystic lesions, they have a decreased density and merge with the edema area on CT images. In cases of hemorrhagic inclusions or calcifications, breast cancer metastases have an increased density on CT before contrast enhancement. The deposition of calcium in the structures of metastases is more common during the treatment (chemotherapy or radiotherapy); therefore, the detection of calcifications in a long-term disease may indicate that they belong to the “old,” not newly diagnosed metastases.
The majority of breast cancer metastases affecting the skull bones (47–85%) are characterized by an osteolytic form, although they can grow as osteoblastic metastases characterized by an increased density.
The contrast agent administration on CT allows to identify a solid part of breast cancer metastases in the brain. Small (<5 mm) metastatic lesions often accompanying the larger ones can remain undetected even with contrast enhancement.
On CT perfusion, a solid part of breast cancer metastases is characterized by an average blood volume CBV of 11.57 ± 5.79 ml/100 g with a large individual variation of the parameter values, close to the respective values of metastases of lung cancer, colorectal cancer, and uterine cancer . The parameter of blood flow velocity CBF has quite high values (92.04 ± 29.93 ml/100 g/min), also with a large dispersion of individual values. Average values of MTT parameter characterizing the blood transit through the capillary network of the metastasis were 7.6 ± 3.11 s. These perfusion parameters of metastatic breast cancer may provide important additional information required for the differential diagnosis of primary and secondary focal brain lesions, as well as metastatic lesions from primary tumors with various locations.
An MR study of suspected metastatic brain lesions in breast cancer is performed using the classical protocol. The nature of the CA accumulation by breast cancer metastases in our materials was diverse: along with homogeneous accumulation of the contrast agent by the solid part of the tumor, lesions with heterogeneous accumulation of the contrast agent were identified. In the majority of cases, after the contrast enhancement, a marked increase in the intensity of the MR signal from the tumor was noted, which facilitated the identification of sites and number of lesions.
The quality and reliability of visualization of metastases located in the posterior fossa, in the basal temporal region, and in convexital portions of the cerebral hemispheres are improving on T1-weighted MRI with fat signal suppression.
According to our observations, breast cancer is accompanied by significant involvement of the meninges (50% of cases), with the possible involvement of leptomeninges only. In cases of suspected metastases in the meninges, it is very important to use T1-weighted MRI with fat suppression and intravenous CA administration. Isolated metastases can spread through the dura mater and simulate meningioma, for example, if located at the base of the brain. Therefore, a differential diagnosis with the primary tumor or a non-tumor lesion must be carried out quite often for individual intracranial metastases of breast cancer.
Rarely breast cancer metastases in the brain can be detected at untypical sites, for example, in the sellar cavity. Metastases to the pituitary affect its posterior lobe and stalk more often than the anterior lobe, which can be associated with the direct blood supply to the neurohypophysis. About 35% of breast cancer metastases are located in the posterior cranial fossa, in the cerebellar peduncle, becoming multiple in some cases.
In our material, there are cases of patients with metastatic involvement of the orbit (6%), and, which is not typical for “untreated” metastases, tumor lesions contained small calcifications clearly detectable on CT. In metastatic involvement of the orbit, the uvea, the retrobulbar space, and its bony walls are often affected. Metastases of breast cancer in the orbit have diffuse and heterogeneous growth, whereas metastases of, for example, clear cell renal cell carcinoma and melanoma have clearly defined boundaries.
According to several authors, in adults, the primary source of metastases to the orbit is lung adenocarcinoma, breast cancer , prostate cancer, myeloma, lymphoma, and neuroblastoma (Lieb 2010). Malignant tumors of the orbit in 34% of cases are of metastatic origin or grow into the orbit from the adjacent areas. Women are almost two times more likely to get affected (Henderson et al. 1993).
On SWI (SWAN) MRI, breast cancer metastases in the brain have similar manifestations to those of lung cancer metastases, with a small number of hypointense inclusions in their structure. In the presence of hemorrhagic inclusions after radiotherapy, the use of standard MR protocols does not allow to reliably exclude the presence of a residual tumor. The combination of quantitative parameters of the coefficient of signal uniformity (CSU) on SWI (SWAN) maps and values of CT perfusion allows to obtain more accurate information about changes that reflect the “normal” course of post-radiation pathomorphism. After radiosurgical treatment, a pronounced accumulation of CA in the area of the irradiated metastasis can persist, which looks like a large hypointense area on SWI (SWAN) images, corresponding to the post-radiation changes (vasculitis).
On ASL maps in solid metastatic foci, even in the presence of pronounced hemorrhages, there is an increase in perfusion parameters. However, if the solid part is small (up to 1 cm), breast cancer metastases are difficult to differentiate on the background of the brain substance.
MR spectroscopy in breast cancer showed no reliable specificity. Changes similar to PMR spectra characteristic of metastatic lesions are also observed in glioblastoma and other tumors with necrotic decay.
The use of PET with 18F-FDG for the primary diagnosis of breast cancer is not advisable. Today a series of activities is carried out aimed at finding specific radiopharmaceuticals to identify the “sentinel” lymph node. One of the most common drugs is 18F-FES (fluoroestradiol): the drug is sensitive to ER+ receptors and can be used to assess the effectiveness of hormone therapy in breast cancer. A significant reduction in the accumulation of 18F-FES in breast cancer metastases is noted following hormone therapy and, importantly, in the menopause period. Some patients may simultaneously have ER+ and ER− metastases, making it difficult to visualize tumor lesions (Vaalavirta et al. 2014).
Using whole-body PET as the first diagnostic step allowed in some cases to eliminate the need in ultrasound, CT, MRI, endoscopy, scintigraphy, and other study methods for a search of the primary tumor and, thereby, significantly reduce the time and costs of diagnostic activities. However, even upon identifying clear areas of abnormal CA accumulation on PET, the morphological verification of lesions is required.
13.1 Clinical Cases
See Figs. 13.1, 13.2, 13.3, 13.4, 13.5, 13.6, 13.7, 13.8, 13.9, 13.10, 13.11, 13.12, 13.13, 13.14, 13.15, 13.16, 13.17, 13.18, 13.19, 13.20, 13.21, 13.22, 13.23, 13.24, 13.25, 13.26, 13.27, 13.28, and 13.29.
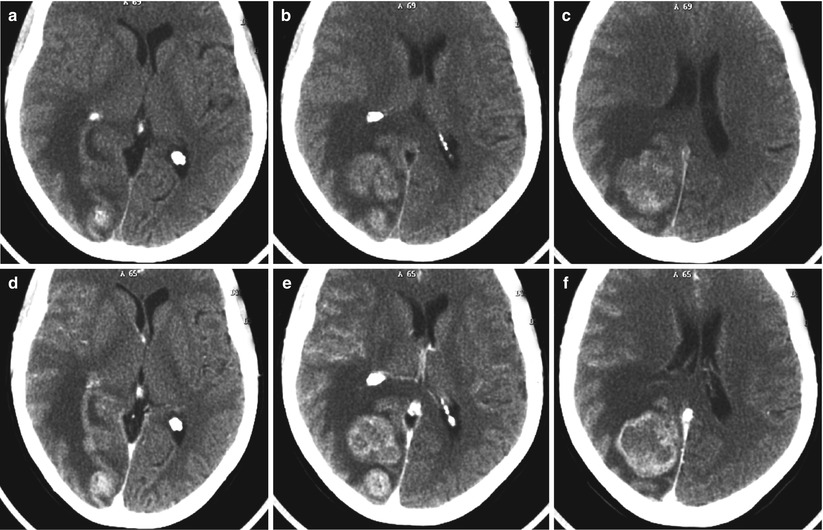
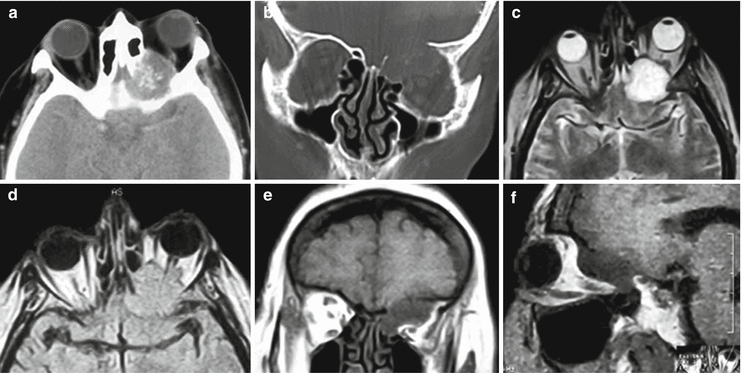
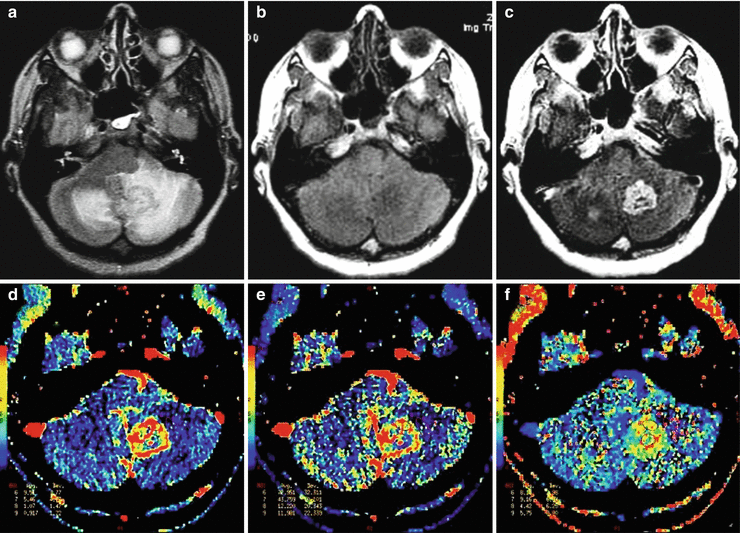
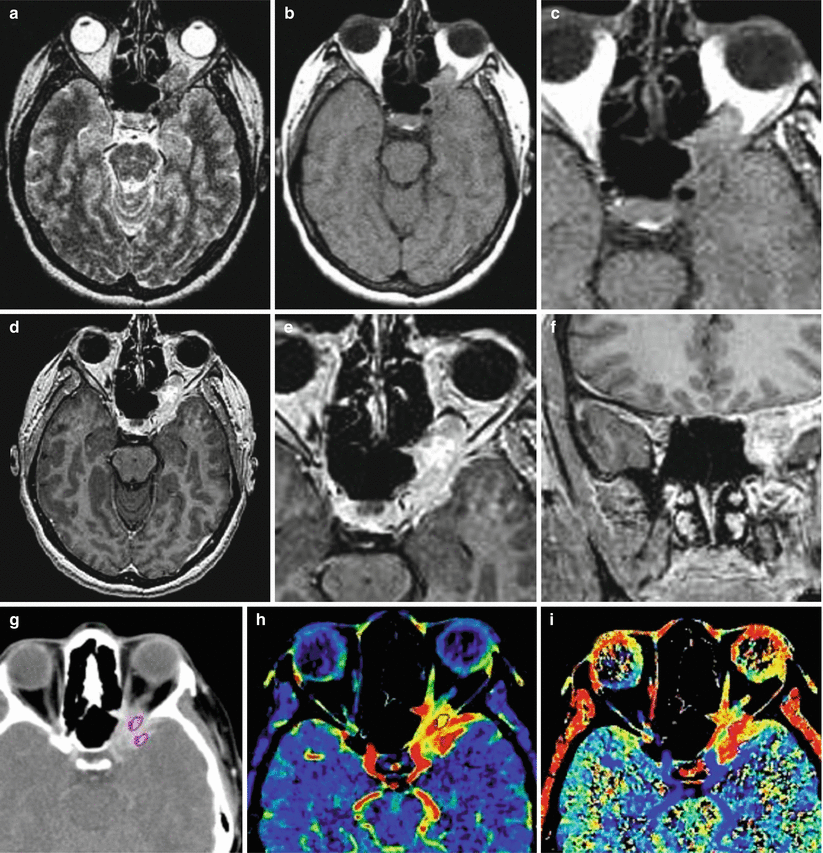
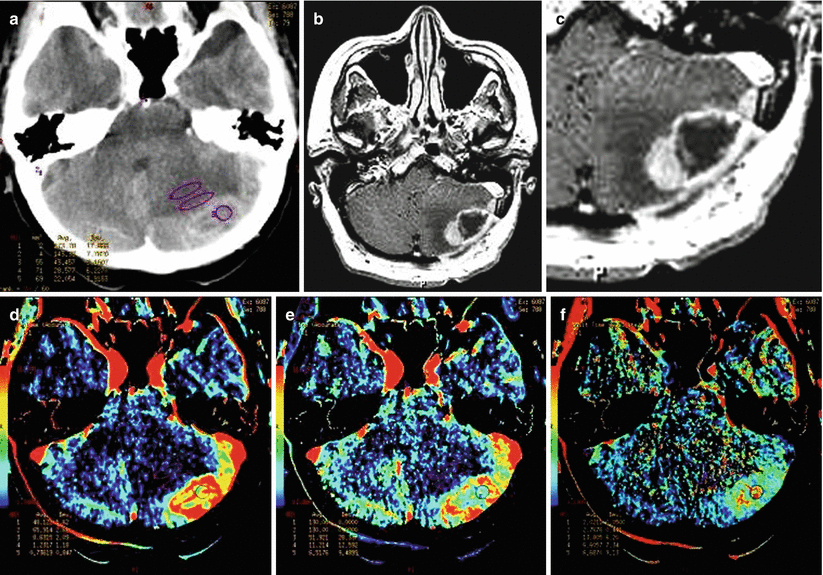
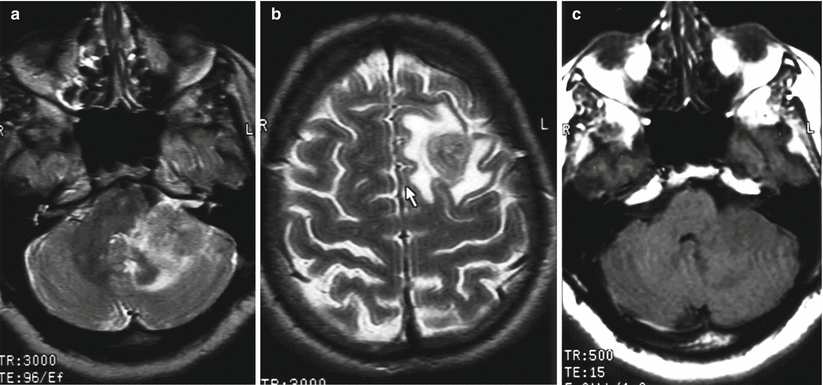
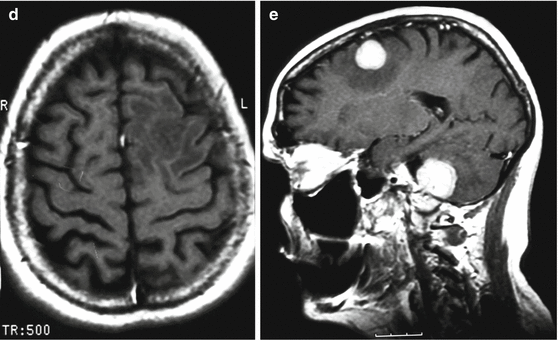
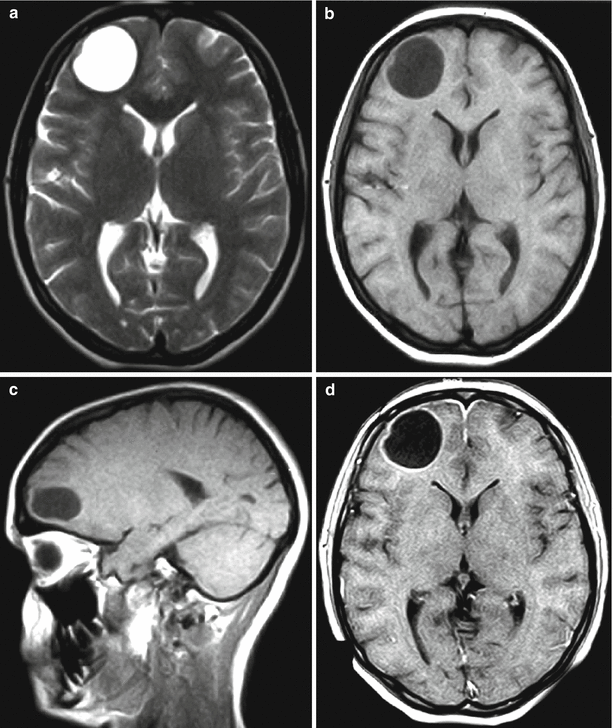
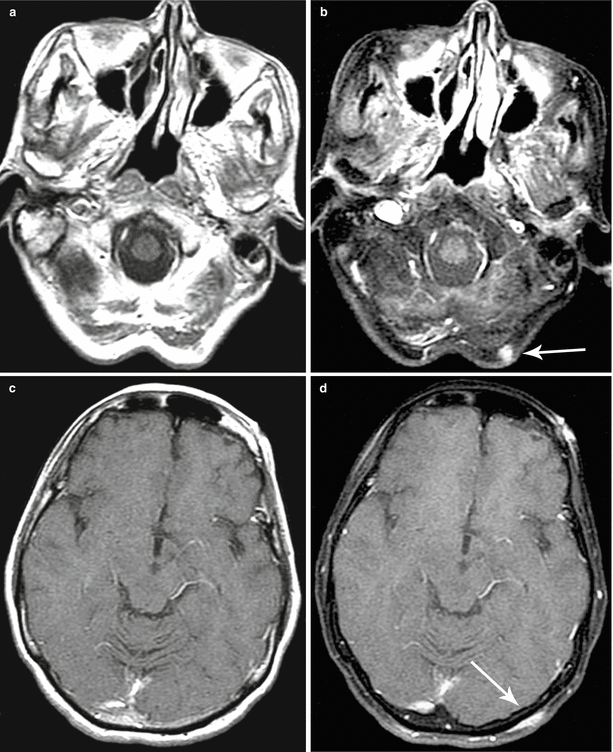
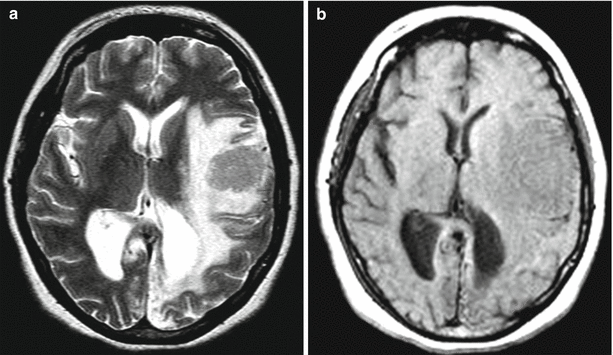
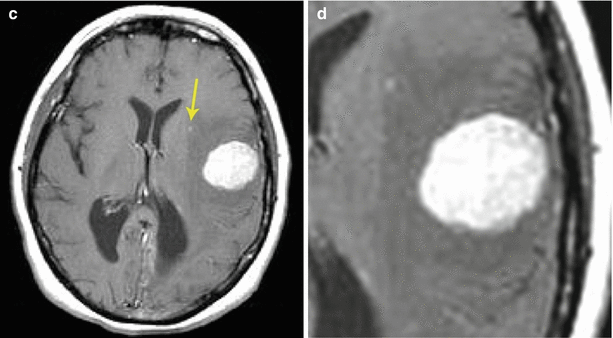
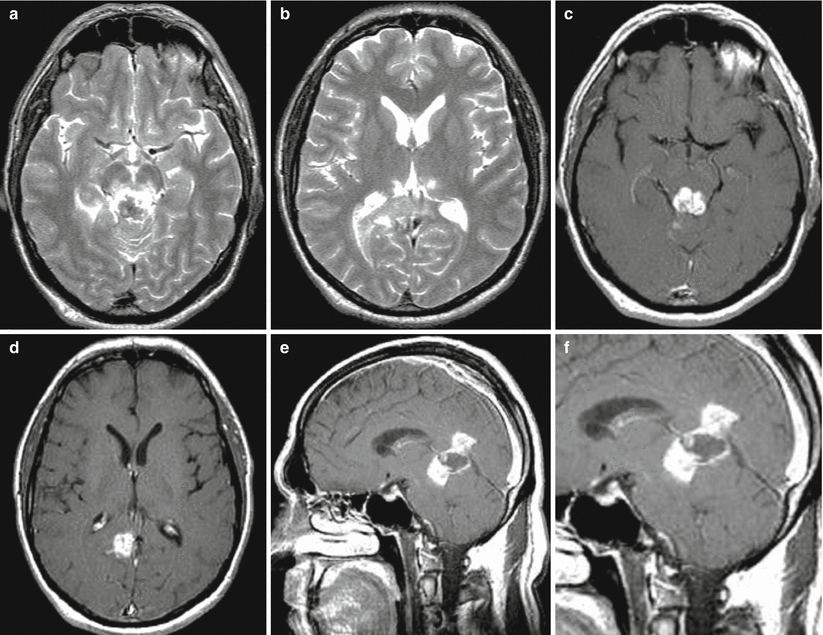

Fig. 13.1
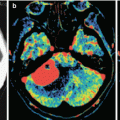
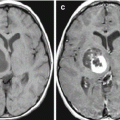
Multiple breast cancer metastases in the brain. CT before (a–c) and after (d–f) intravenous contrast enhancement. On the background of edema, multiple tumor lesions with a solid structure and a marked contrast enhancement are determined. The ventricular system is deformed

Fig. 13.2
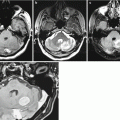
A breast cancer metastasis. On CT in axial (a) and front (b) projections, an irregularly shaped tumor in the left orbital funnel is identified, causing the destruction of the orbital walls. There are small calcifications in the center of the tumor. On axial T2-weighted MRI (c) and T2-FLAIR (d) MRI, a quite homogeneous tumor is well defined. T1-weighted MR images in the frontal (e) and sagittal (f) views clearly show the destruction of the upper orbital wall and compression of the orbital fat and the optic nerve

Fig. 13.3
Multiple breast cancer metastases in the brain. On MR images, in the left and right hemispheres of the cerebellum, there are areas with an iso-hyperintense signal on T2-FLAIR MRI (a) and a moderately hypointense signal on T1-weighted MRI (b). After the CA administration, its uneven intense accumulation is observed in a large lesion on the left and in a small lesion on the right (c). On CT perfusion maps, there is a marked increase in all perfusion parameters: CBF (d), CBV (e), and MTT (f)

Fig. 13.4
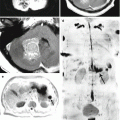
A breast cancer metastasis. In the retrobulbar space on the left, there is a lesion with a low signal on T2-weighted MRI (a) and an isointense signal on T1-weighted MRI (b, c) with the spread to the orbital funnel. The lesion intensely accumulates CA (d–f), while the part of the tumor extending to the dura mater along the large wing of the sphenoid bone is well visualized. On contrast-enhanced CT (g), the tumor also intensely accumulates CA. On CT perfusion maps, the CBV (h) and MTT (i) values are increased both in the intraorbital portion of the tumor and along the meninges of the left temporal region

Fig. 13.5
A breast cancer metastasis in the brain. There is a solid-cystic lesion unevenly accumulating CA both on CT (a) and T1-weighted MRI (b, c) with signs of a moderate perifocal edema in the left hemisphere of the cerebellum. The fourth ventricle is compressed, and the brainstem is deformed. On CT perfusion, increased values of CBV (d), CBF (e), and MTT (f) are observed in the solid part of the metastasis


Fig. 13.6
Multiple breast cancer metastases in the brain. On T2-weighted (a, b) and T1-weighted (c, d) MRI, two solid tumor lesions are visualized supra- and subtentorially, which have low hyperintensity on T2 and low hypointensity on T1 signal intensity. The contrast enhancement is expressed and homogeneous (e)

Fig. 13.7
A breast cancer metastasis in the brain. On MR images in the right frontal region, there is a large cystic lesion without a perifocal edema (a–c). After administration of the contrast agent, its intense accumulation is noted along the lesion contour in the form of a thin rim (d)

Fig. 13.8
Multiple breast cancer metastases in the brain. A two-level study. On T1-weighted MRI with contrast enhancement (a, c), no conclusive evidence of abnormalities have been detected. On T1-weighted MRI supplemented with fat suppression protocol (b, d), extracranially, in the soft tissue of the occipital region and the left occipital-parietal region, visualize lesions accumulating the contrast agent (the arrows)


Fig. 13.9
A breast cancer metastasis in the brain. In the left frontotemporal region, there is a space-occupying solid lesion with an isointense MR signal on T2-weighted MRI (a) and moderately hypointense on T1-weighted MRI (b) with signs of a pronounced perifocal edema. The lesion intensely and uniformly accumulates the contrast agent (c, d). A pinpoint portion of accumulation medially to the main lesion is likely a blood vessel (c) (the arrow). There is displacement of the midline structures to the right and a moderate compression of the left lateral ventricle

Fig. 13.10




A breast cancer metastasis. A two-level study. In the pineal region, there is an area with an iso-hyperintense MR signal on T2-weighted MRI without clear contours (a, b). After the CA administration, there is its intense uneven accumulation (c, d) in the specified area. In the sagittal projection, the upper and lower solid components of the tumor, as well as the central part of the cystic metastasis (e, f) are well visualized
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree