Acknowledgments
Funding was provided in part by the 2020–Research and Innovation Framework Program PHC-11-2015 Nr. 667211, Jubiläumsfonds of the Austrian National Bank # Nr: 18207and seed grants from Novomed Austria and Guerbet France.
Katja Pinker is funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and the Breast Cancer Research Foundation.
Introduction
Breast cancer is a heterogeneous disease, presenting with specific molecular patterns that differ in terms of biological behavior and prognosis and thus allowing for the possibility of targeted treatments. During carcinogenesis, cells progressively acquire capabilities to avoid apoptosis, generate new highly permeable vessels and replicate in an uncontrolled manner; these newly acquired characteristics have been described as the “hallmarks of cancer” ( ). The need to understand the biological properties of tumors at the cellular level, in order to assess their aggressiveness and particularly to predict their response to a specific treatment, has prompted the development of functional and molecular imaging techniques that can noninvasively depict such biological properties. Among these, magnetic resonance imaging (MRI) is a mainstay in breast imaging, being routinely performed in clinical practice according to well-established clinical indications ( ); it is also able to provide functional data through the implementation of advanced sequences such as diffusion and perfusion-weighted imaging as well as magnetic resonance spectroscopy (MRS) within a multiparametric approach ( ). MRI is the most accurate imaging modality for breast cancer diagnosis, with a reported sensitivity and specificity of 99% and 89%, respectively, and for the assessment of the response to neoadjuvant chemotherapy (NAC) ( ; ). Positron emission tomography (PET) imaging, performed in clinical practice as hybrid 2-deoxy-2- 18 F fluoro- d -glucose ( 18 F-FDG) PET/computed tomography (PET/CT), holds a leading role in the evaluation of patients with breast cancer, from staging and assessment of response to treatment, to the detection of tumor recurrence ( ). Considering the excellent resolution of MRI in the breast parenchyma, it is not surprising that following the introduction of the hybrid PET/MRI scanner in 2011, the use of simultaneous PET/MRI in patients with breast cancer has been extensively investigated ( ; ; ; ; , ). In this chapter, state-of-the-art breast PET/MRI will be illustrated, from imaging acquisition to the main clinical applications.
Breast PET/MRI acquisition technique
Acquisition protocol
Breast 18 F-FDG PET/MRI
For the purposes of PET acquisition, patients should fast for at least 5–6 h before the start of image acquisition. A total of 2.5–3.5 MBq/kg body weight of 18 F-FDG is injected intravenously approximately 60 min before PET/MRI acquisition. Blood glucose levels are checked before injection and should not exceed 150 mg/dL (8.3 mmol/L).
As PET detectors are fully integrated into the MRI scanner, simultaneous PET/MRI of the breast is performed as a routine MRI examination, with the patient lying in the prone position, both breasts positioned in the dedicated breast coils and arms positioned over the head. A warm blanket can be used before and during PET/MRI acquisition to alleviate patient discomfort and to avoid brown fat activation due to the low indoor temperature ( ). MRI acquisition starts with localization and morphological sequences such as T2-weighted imaging with and without fat suppression. Functional sequences, that is, diffusion-weighted imaging (DWI) with corresponding apparent diffusion coefficient (ADC) maps are then acquired. Subsequently, pre- and postcontrast dynamic sequences with high temporal resolution are acquired before and after gadolinium injection trough the antecubital vein. Precontrast T1-mapping with different flip angles is recommended for the calculation of quantitative perfusion parameters. A high-resolution postcontrast T1-weighted sequence can finally be acquired to provide superior anatomical details, as compared with that provided by lower-resolution postcontrast dynamic sequences. The preferred acquisition plane is the axial one, especially for dynamic isovolumetric acquisitions, but coronal and sagittal planes can also be employed. PET data are typically acquired at the beginning of the MRI acquisition for approximately 2–15 min. The total acquisition time is around 20 min.
Whole-body 18 F-FDG PET/MRI
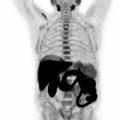
The patient is placed in the supine position, with head and body coils. If whole-body PET/MRI is performed in combination with dedicated breast PET/MRI, no additional radiopharmaceuticals/gadolinium are injected after the ones used for the initial PET/MRI breast examination. The whole-body protocol typically includes coronal T2-weighted sequences, with and/or without fat suppression, an axial DWI sequence and a coronal high-resolution fat-saturated T1-weighted sequence for each bed position. Single bed position data are then combined to obtain whole-body images ( Fig. 10.1 ). Similar to breast acquisition, PET data are acquired during MRI acquisition. The total acquisition time is around 30–40 min.

Tracers
While different radiopharmaceuticals are currently available for the molecular imaging of breast cancer, only two have been approved by the United States Food and Drug Administration, namely, 18 F-FDG and fluorine 18-sodium fluoride ( 18 F-NaF). 18 F-FDG is widely adopted for several clinical indications from staging to follow-up. It has some limitations, including high uptake in dense breast parenchyma and, conversely, low FDG uptake can occur in lobular carcinoma and small subcentimeter breast cancer lesions. 18 F-NaF has been used for the detection of bone metastases, having demonstrated a higher sensitivity compared with 18 F-FDG and bone scintigraphy albeit with a lower specificity ( ). However, its use in clinical practice is limited due to its high cost-effectiveness ratio compared with routinely used imaging techniques. Interestingly, PET tracers have been developed to detect estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2), such as fluorine 18F-Fluoroestradiol ( 18 F-FES) and zirconium-89-Trastuzumab ( 89 Zr-Trastuzumab), respectively ( ; ). However, despite promising initial findings, no cut-off values have as yet been established for the assessment of 18 F-FES uptake; moreover, in the case of 89 Zr-Trastuzumab, its long half-life requires multiple and/or late time point acquisition, limiting its use in clinical practice. Other tracers under investigation for molecular imaging of breast cancer are 18 F-fluorothymidine for the assessment of cell proliferation and 89 Zr-atezolizumab for the evaluation of PD-L1 expression ( ). Recently, the possible use of fluorine 18 ( 18 F)-choline and carbon 11 ( 11 C)-choline has also been explored due to their ability to assess cell proliferation and metabolism, especially considering that elevated choline peaks have been reported in breast cancer lesions on MRS ( ; ).
Imaging
Breast cancer staging
MRI has well-recognized clinical indications. Particularly, it is indicated for preoperative staging of breast cancer, to accurately define tumor extension, to detect multifocality/centricity, and for screening of the contralateral breast ( ). It has also been shown to be superior to conventional imaging for the evaluation of tumor response to NAC. At present, 18 F-FDG PET/CT is recommended for staging patients with clinical stage II B breast cancer or greater ( ). It is considered optional for patients with clinical stage II A breast cancer or greater and not recommended for stage I breast cancer or for the assessment of T status. According to the recently updated National Comprehensive Cancer Network guidelines, 18 F-FDG PET/CT is not indicated for clinical stages I, II or operable III breast cancer. This is due to the high false-negative rate in small or low-grade breast cancer lesions, the low sensitivity of detecting lymph node metastases as well as the low probability of distant metastases, potentially resulting in false positive findings ( ; ). The use of 18 F-FDG PET/CT can result in a significant change in patient clinical management, which mainly occurs when distant metastases are detected, that shifts the clinical management toward systemic treatment. Furthermore, the possibility of detecting an unexpected solitary metastasis can allow the performance of localized therapy (i.e., radiotherapy), which might improve the chances of survival.
Fervent research activity has been conducted to further define the clinical indications of 18 F-FDG PET/CT during the initial stage of breast cancer and especially to assess the added value of 18 F-FDG PET/MRI due to its greater contrast resolution in breast tissue.
Several studies demonstrated a superior performance of 18 F-FDG PET/MRI versus 18 F-FDG PET/CT in whole body staging of breast cancer ( ; ). A systematic review and meta-analysis including 29 studies with a total of 4276 patients demonstrated the high impact of 18 F-FDG FDG-PET, 18 F-FDG PET/CT and 18 F-FDG PET/MRI on clinical staging and clinical management of patients with breast cancer. As expected, the rate of stage changes was proportional to the initial stage of breast cancer. Indeed, the pooled proportion of stage changes was 11% in initial stage I, 20% in initial stage II and 34% in initial stage III breast cancer ( ). When analyzing clinical management changes, the authors reported that these were mainly due to additional findings detected by PET. While the findings are in agreement with the basis for current clinical guidelines that PET imaging has a higher impact among patients with higher stage breast cancer (i.e., stage 3), in a nonnegligible portion of stage II and I cases, treatment planning was changed after PET examination.
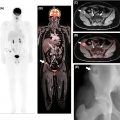
18 F-FDG PET/MRI also proved to have a considerable impact when staging patients who were candidates for NAC in a prospective study by Goorts et al. who specifically assessed its added clinical value over conventional imaging. According to their findings, 18 F-FDG PET/MRI changed treatment planning in 10% of 40 patients with primary invasive T2-4 N0 or T1-4 N+ breast cancer, due to the detection of lymphadenopathy and bone metastases ( ) ( Fig. 10.2 ).
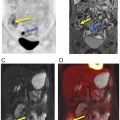
A study by Sawicki and colleagues directly compared the performance of 18 F-FDG PET/MRI versus 18 F-FDG PET/CT, MRI, and CT alone in whole-body staging of recurrent breast cancer in 21 patients. According to the reference standard given by histopathological results and follow-up imaging, only 18 F-FDG PET/MRI detected all cases of breast cancer recurrence; correspondingly, it had the highest diagnostic performance compared with other imaging techniques ( ).
A more recent study was performed to compare the accuracy of 18 F-FDG PET/MRI, MRI, CT and bone scintigraphy for the detection of bone metastasis in the initial staging of breast cancer. In a prospective cohort of 154 patients, in which seven (4.5%) patients had 41 bone metastases, both 18 F-FDG PET/MRI and MRI were able to detect all lesions with 100% sensitivity and specificity. Conversely, 23 and 15 lesions were detected by CT (sensitivity: 71.4%, specificity: 98.6%) and bone scintigraphy (sensitivity: 28.6%, specificity: 99.4%), respectively ( ) ( Fig. 10.3 ). This reinforces a previous study that demonstrated 18 F-FDG-PET/MR being able to detect osseous metastases in 12% of patients deemed negative at same day 18 F-FDG-PET/CT ( ).

Lately, a meta-analysis was carried out to assess the value of 18 F-FDG PET/MRI in TNM assessment of breast cancer patients, and included 20 studies with a total of 666 patients. The pooled sensitivity, specificity, and area under the curve (AUC) of 18 F-FDG PET/MRI were 91%, 91%, and 0.96 for the diagnosis of the T stage; 94%, 90%, and 0.96 for the N stage; and 98%, 96%, and 0.99 for the M stage, respectively ( ). Such promising findings can be explained as follows: MRI can be empowered by 18 F-FDG PET for the visual assessment of breast lesions and lymph node metastases, while vice versa, bone metastases with faint FDG uptake on 18 F-FDG PET, such as lytic and permeative, can be detected by MRI ( ). Furthermore, 18 F-FDG PET/MRI offers the unique opportunity to perform a single-shot imaging examination for the assessment of T, N and M parameters, having demonstrated a remarkable usefulness in locally advanced breast cancer for the assessment of lymph node and distant metastases.
Despite such promising findings, further high-level evidence is needed to confirm the added value of PET/MRI over PET/CT and, particularly, cost-effectiveness analyses should be carried out to promote the inclusion of such an expensive and less available technique in the clinical guidelines.
Breast cancer diagnosis (benign vs. malignant)
At present, the use of 18 F-FDG PET/CT is not recommended for breast cancer diagnosis. However, its use for the noninvasive functional assessment of breast lesion biology (metabolism, neoangiogenesis, permeability, and cellularity) is compelling and has been investigated over the last years.
Most of the published studies on the use of 18 F-FDG PET/CT for breast cancer diagnosis have been conducted on separately acquired PET and MRI images. Magometschnigg and colleagues compared the diagnostic accuracy of prone 18 F-FDG PET/CT with MRI performed for the purpose of evaluating suspicious breast lesions in 172 patients ( ). Both techniques performed equally, with a diagnostic accuracy of 93%, albeit MRI seemed to be more sensitive but less specific than 18 F-FDG PET/CT in the assessment of subcentimeter lesions.
The combined value of different combinations of 18 F-FDG PET/CT, dynamic contrast-enhanced MRI, DWI, and MRS was investigated by Pinker and colleagues to improve the differentiation of 23 benign and 53 malignant breast lesions ( ). The multiparametric approach including all modalities achieved a significantly higher accuracy (AUC = 0.935) compared with other combinations and would have resulted in a reduction of unnecessary breast biopsies.
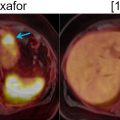
Similarly, Bitencourt et al. applied a multiparametric DCE-MRI, DWI and 18 F-FDG PET/CT approach for evaluating breast lesions, finding a sensitivity, specificity and accuracy of 100%, 55.5% and 89.5%, respectively ( ), Fig. 10.4 . The authors concluded that multiparametric 18 F-FDG PET and MRI functional data could reduce the number of unnecessary biopsies.

The possibility of calculating quantitative perfusion parameters describing lesion permeability (supposed to be increased in the malignant ones), such as Ktrans, Ve and Kep, extracted from high temporal resolution DCE-MRI within a simultaneous 18F-FDG PET/MRI protocol, proved to be feasible and accurate for breast cancer diagnosis in a study sample of 98 women with 109 breast lesions ( ).
Interestingly, in another study, PET/MRI features of contralateral healthy tissues, that is, background parenchymal enhancement, background parenchymal uptake and fibroglandular tissue were found to be different in a study by Leithner and colleagues and may be potentially used as imaging biomarkers for the presence of malignancy ( ).
Overall, the current evidence suggest that hybrid 18 F-FDG PET/MRI imaging has the potential to noninvasively describe the biological properties of breast lesions and thus discriminate benign lesions from the malignant ones. Particularly, the addition of MRI may improve the accuracy of PET which is currently impaired by high false-positive findings ( ).
Tumor characterization
The rationale of functional imaging is to noninvasively detect the features of the tumor that are related to its biological behavior, in order to establish the most appropriate treatment and predict the prognosis of the disease. Cellularity, neoangiogenesis and metabolism as detected by 18 F-FDG PET/MRI through the extraction of quantitative data can shed light on tumor aggressiveness. Indeed, several studies have investigated and found relationships between 18 F-FDG PET/MRI metrics and tumor histological markers ( ) such as Ki67 expression (Jena, Taneja, Singh, Negi, Sarin, et al., 2017b; ), metastatic burden ( ), and invasive ductal carcinoma phenotypes ( ; ).
More recently, 18 F-FDG PET/MRI parameters have been correlated with the level of tumor infiltrating lymphocytes (TILs), reflecting antitumor cell activity, in 55 women with triple negative or HER2 positive invasive ductal carcinoma ( ). High maximum standard uptake values (SUVmax) were found in patients with high and intermediate TILs levels as well as in patients with low TILs levels and peritumoral edema. These findings suggest that 18 F-FDG PET/MRI may provide important preoperative information for treatment planning.
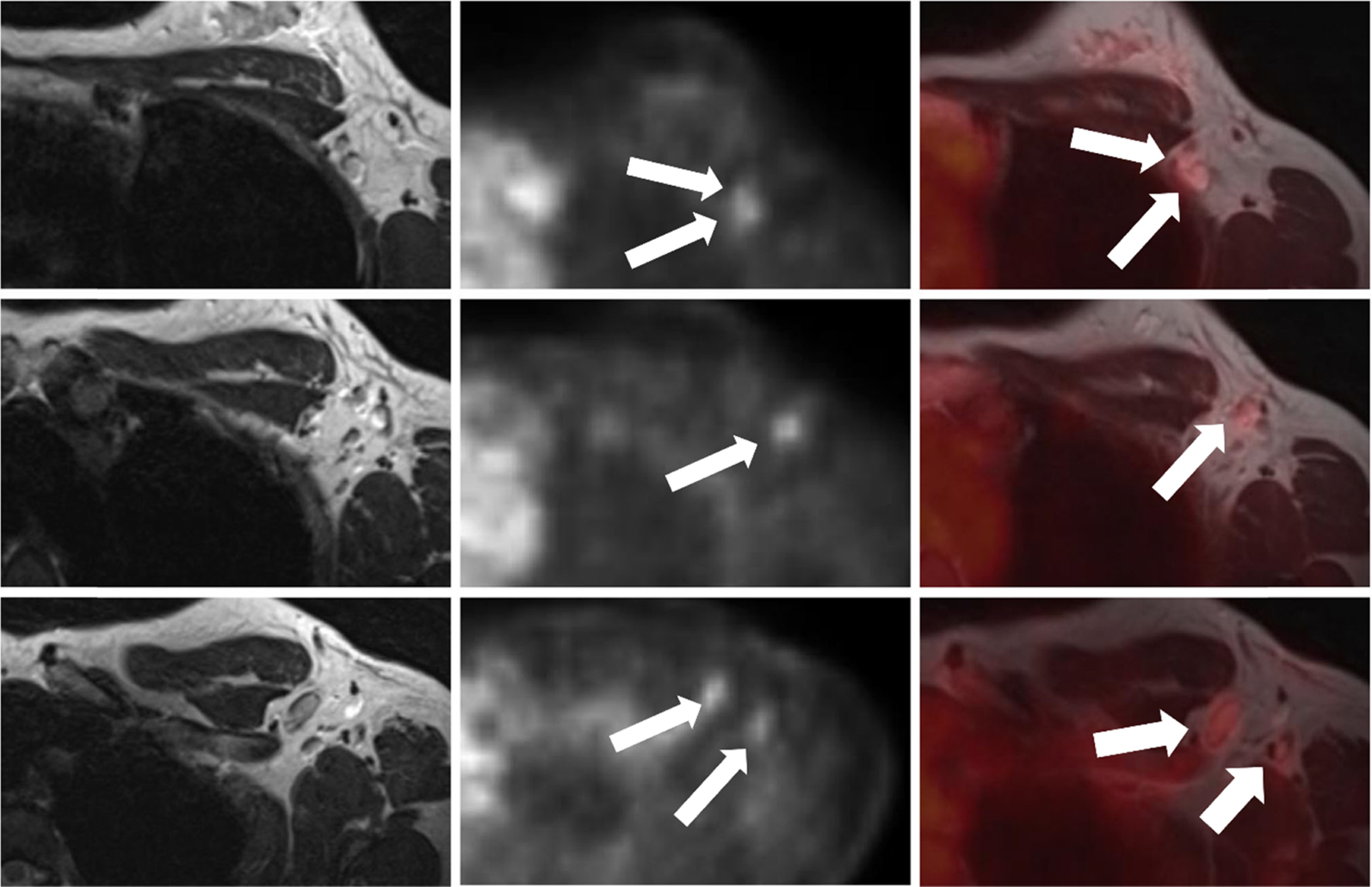
Tracers other than 18 F-FDG have also been employed in PET/MRI imaging for the evaluation of tumor hypoxia, such as 18F-fluoromisonidazole ( 18 F-MISO). A study by Andrzejewski and colleagues assessed the feasibility of sequential 18 F-FDG/ 18 F-MISO PET/MRI for the evaluation of breast cancer heterogeneity. The authors found several intermediate and strong positive correlations between imaging biomarkers and histopathologic tumor features such as proliferation rate, whereas ER expression was correlated negatively with PET biomarkers ( ) ( Fig. 10.5 ).
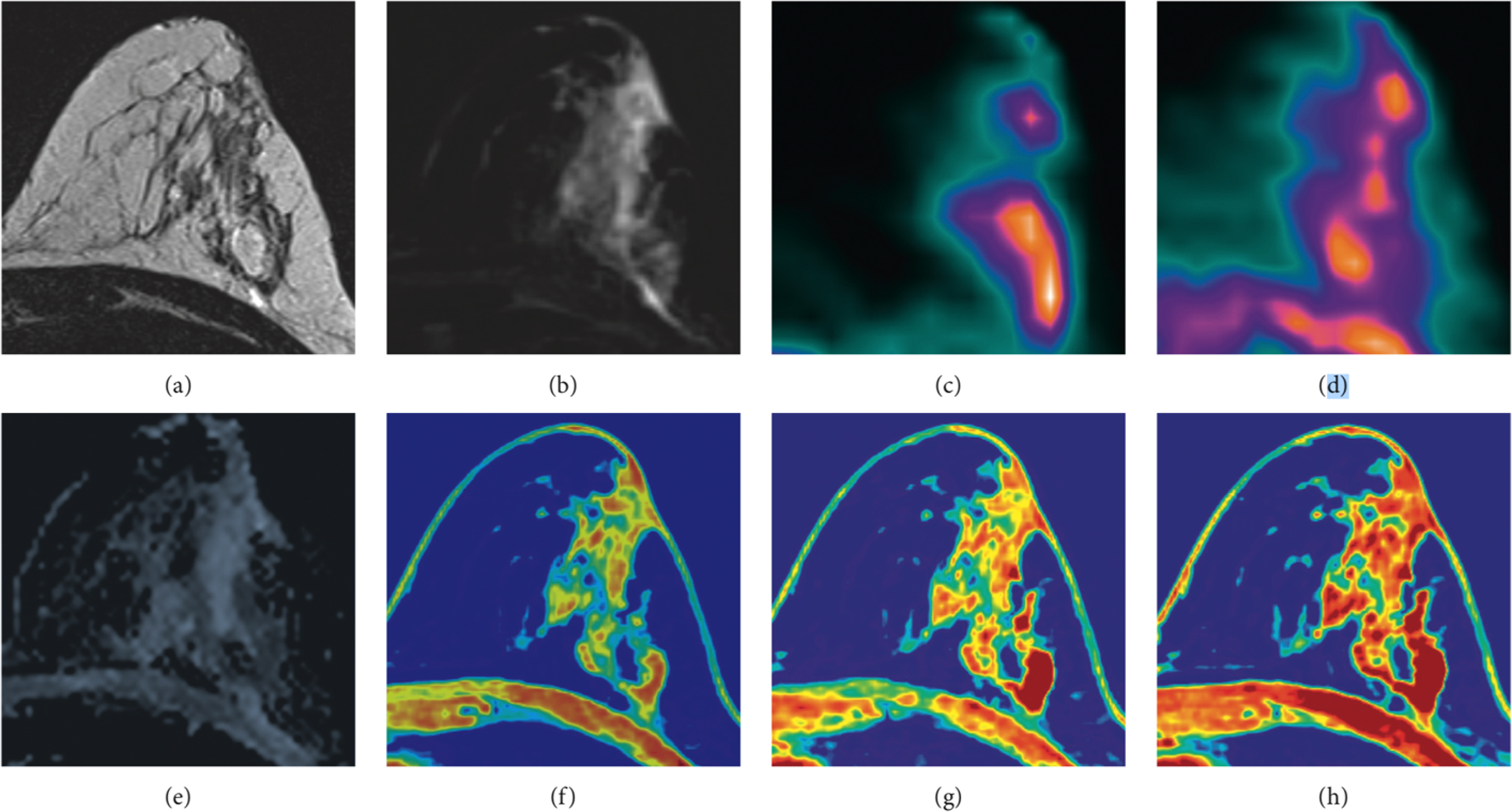
In a more recent investigation, the relationship between hypoxia, cellularity and neoangiogenesis was explored in 29 women with 32 breast cancer lesions using 18 F-MISO PET/MRI through the calculation of the influx rate constant (Ki), the hypoxic fraction (%HF), tumor ADC and quantitative perfusion parameters (Ktrans, Kep, Ve, Vp) ( ), as shown in Fig. 10.6 . A negative correlation was found between Ktrans and Ki and %HF, although hypoxic regions colocalized with both hyper and hypo-perfused tumor areas on multiparametric perfusion maps. Interestingly, %HF correlated positively with tumor size. These findings suggest, as expected, that hypoxia occurs in less perfused/permeable tumor areas as well as in large, rapidly growing, cancer lesions.