There is no difference in disease-free or overall survival in patients who undergo adjuvant versus neoadjuvant chemotherapy. Thus, neoadjuvant chemotherapy is recommended in patients with locally advanced breast cancer who would like to consider breast conservation, and is also the primary treatment in patients with inflammatory breast cancer. Magnetic resonance has emerged as the most sensitive imaging modality to assess the response of tumor to neoadjuvant chemotherapy.
Key points
- •
Dynamic contrast-enhanced (DCE) breast magnetic resonance (MR) is the most sensitive imaging modality in routine clinical practice and the most highly correlated with disease.
- •
The information obtained from DCE-MR is of clinical usefulness in guiding oncologists in treatment assessment and in guiding surgeons in the selection of patients who are potential candidates for breast conservation.
- •
The type of response to neoadjuvant chemotherapy noted by DCE-MR may be a predictor of overall and disease-free survival.
- •
Although there may be false-negative appearances caused by the chemotherapy effect on neovascularity.
Introduction
Dynamic contrast-enhanced breast magnetic resonance (MR) imaging (DCE-MR) is now recognized as an important adjunct imaging modality in the evaluation of patients with new diagnosis of breast cancer. The American College of Radiology has created and revised the guidelines for the performance of DCE-MR. Related to breast cancer imaging, the indications include evaluation of extent of disease for a known cancer, screening of high-risk patients, screening of the contralateral breast for patients with a new breast malignancy, and assessing response to neoadjuvant chemotherapy. The indications for neoadjuvant chemotherapy include decreasing the size of tumor to render the patient a candidate for breast conservation, predicting long-term disease-free survival, and primary treatment of inflammatory breast cancer. This article reviews locally advanced breast cancer and the role DCE-MR plays in its management, as well as emerging MR technology specific to assessment of response to neoadjuvant chemotherapy.
Introduction
Dynamic contrast-enhanced breast magnetic resonance (MR) imaging (DCE-MR) is now recognized as an important adjunct imaging modality in the evaluation of patients with new diagnosis of breast cancer. The American College of Radiology has created and revised the guidelines for the performance of DCE-MR. Related to breast cancer imaging, the indications include evaluation of extent of disease for a known cancer, screening of high-risk patients, screening of the contralateral breast for patients with a new breast malignancy, and assessing response to neoadjuvant chemotherapy. The indications for neoadjuvant chemotherapy include decreasing the size of tumor to render the patient a candidate for breast conservation, predicting long-term disease-free survival, and primary treatment of inflammatory breast cancer. This article reviews locally advanced breast cancer and the role DCE-MR plays in its management, as well as emerging MR technology specific to assessment of response to neoadjuvant chemotherapy.
Locally advanced breast cancer
Box 1 summarizes the characteristics of locally advanced breast cancer. Locally advanced breast cancer is stage III disease that includes large primary tumors or suspicious regional lymph nodes. Primary tumors are considered advanced if they measure greater than 5 cm in greatest dimension, they extend to the chest wall or skin, or if the tumor invades the dermal lymphatics in the case of inflammatory breast cancer. Smaller sized primary tumors may also be considered advanced in relation to a small-sized breast.
Locally advanced breast cancer
Greater than 5 cm
Tumors of 3 to 5 cm in small breast
Tumor of any size associated with skin or chest wall involvement
Fixed or matted axillary lymph nodes
Ipsilateral infraclavicular or supraclavicular nodes
Absence of distant metastasis
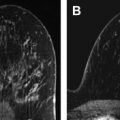
Regional adenopathy is considered advanced if it is level I, II, or III (infraclavicular) axillary adenopathy, ipsilateral supraclavicular adenopathy, or ipsilateral internal mammary adenopathy with or without axillary adenopathy. Nipple retraction and skin changes can also be clinically seen in association with tumors in locally advanced breast cancer. DCE-MR imaging and clinical features of locally advanced breast cancer are shown in Fig. 1 .
Distant metastases are not a feature of locally advanced breast cancer and thus locally advanced breast cancer is amenable to surgical treatment. A distinction is also made between locally advanced breast cancer and inflammatory breast cancer, which are two different diseases. Inflammatory breast cancer is discussed in this article because it is primarily treated with neoadjuvant therapy. In some cases, inflammatory breast cancer can be converted into an operable and curable disease after treatment with neoadjuvant chemotherapy.
Women who undergo routine mammographic screening represent less than 10% of patients with locally advanced breast cancer; however, in many underserved populations and globally, where routine mammographic screening is not available or underused, locally advanced breast cancer can be seen in up to 60% of diagnosed breast cancers. On presentation, locally advanced breast cancers are generally not amenable to breast-conserving surgery. If the woman desires breast conservation, then neoadjuvant chemotherapy may be recommended.
Neoadjuvant versus adjuvant therapy
Neoadjuvant chemotherapy refers to chemotherapy given before surgical therapy. It is also the initial therapy for inflammatory breast cancer. Adjuvant therapy is chemotherapy or hormonal therapy that is given after surgical therapy.
In 1988 the National Surgical Adjuvant Breast and Bowel Project (NSABP) initiated the B-18 randomized clinical trial comparing preoperative and postoperative chemotherapy in patients with operable breast cancer with the goal of finding out whether preoperative chemotherapy improved overall survival and disease-free survival. The study showed that there was no difference in survival, whether overall or disease free, between the two groups using the same chemotherapy regimen. A secondary aim of the study was to evaluate the rates of breast-conserving surgery in the two groups of patients. The study showed that patients who underwent preoperative chemotherapy were more likely to undergo lumpectomy after shrinkage of tumor than those having surgery before chemotherapy.
Box 2 summarizes the rationale for the use of neoadjuvant chemotherapy.
Reduce tumor size to render patient a candidate for lumpectomy
Provides prognostic factors
Time for patient to consider surgical options
Primary treatment of inflammatory breast caner
Protocols for imaging
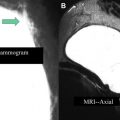
The suggested imaging and treatment protocols for patients with newly diagnosed invasive breast cancer are shown in Fig. 2 . Options include imaging before and after neoadjuvant chemotherapy, and, in some instances, early during chemotherapy. As an alternative, patients may proceed directly to surgery. Imaging is usually performed before and at the conclusion of neoadjuvant chemotherapy, although, as discussed later, there is evidence that diffusion-weighted (DW) MR early during treatment may be able to predict tumor changes, allowing neoadjuvant chemotherapy to be tailored based on response.
Response to neoadjuvant therapy
The Response Criteria in Solid Tumors (RECIST), established by the World Health Organization, is the accepted standard for measuring tumor response to treatment. RECIST categorizes response as complete, partial, stable, or progressive disease based on change in diameter as assessed by imaging or physical examination. For assessment of breast cancer response to neoadjuvant chemotherapy, response is further refined based on histopathologic and immunopathologic evaluation of the surgical specimen. Pathologic complete response (pCR) refers to no residual invasive disease at surgery after completion of neoadjuvant chemotherapy. Depending on the study criteria, pCR may or may not take into account residual in situ disease and/or lymph node status. It is expected that up to 30% of patients undergoing neoadjuvant chemotherapy will achieve pCR. For this reason, placement of marker clips at the time of biopsy of locally advanced breast cancer, regardless of how large the tumor is, is advised to guide breast conserving surgery in this significant group of patients who will achieve pCR.
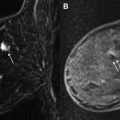
Additional analysis of the NSABP B-18 study determined a worse prognosis for patients with complete clinical response who did not have pCR and whose lymph nodes did not respond to neoadjuvant chemotherapy. Fig. 3 shows DCE-MR imaging appearance in 4 different patients before and after the completion of neoadjuvant chemotherapy showing pCR, partial response, stable disease, and progression of disease.
Lymph node status also provides important prognostic information for disease-free survival and overall survival. Evaluation of regional nodal basin, including the internal mammary lymph node chain, is of clinical importance and should be included in the search pattern evaluation and reporting of multimodality breast imaging. In Fig. 4 , both bulky axillary lymphadenopathy and internal mammary adenopathy were identified in a patient who underwent neoadjuvant chemotherapy. This patient achieved pCR both in the breast and in the lymph nodes.
Because only up to 30% of patients show pCR, a significant number (70% or greater) of patients derive partial or, in some cases, no benefit from neoadjuvant chemotherapy. There has been considerable effort in studying the role of DCE-MR in predicting early response to neoadjuvant chemotherapy. Identifying early markers for response to neoadjuvant chemotherapy could potentially spare patients for whom chemotherapy is ineffective or unnecessarily toxic and would expedite the surgical therapy. The American College of Radiology Imaging Network (ACRIN) study 6657, the imaging component of the Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis (I-SPY TRIAL), is one such multicenter, prospective, randomized breast cancer trial. In this study, patients underwent DCE-MR before, 2 weeks after anthracycline-based chemotherapy, between anthracycline and taxane chemotherapy, and before surgery. Results of ACRIN 6657 have shown that decrease in tumor diameter and tumor volume early during therapy may be the best predictors for pCR.
Fig. 5 shows a suggested algorithm combining the clinical management and imaging assessment of locally advanced breast cancer. A combination of imaging modalities and clinical examination is used to assess the response during and at the completion of neoadjuvant therapy. If the patient is noted to progress early in the course of therapy, a change in chemotherapy regimen or earlier surgical intervention is considered.
Imaging modalities to assess response to neoadjuvant chemotherapy
As described earlier, both clinical assessment and various imaging modalities can be used to evaluate patients with locally advanced breast cancer and assess the response to neoadjuvant chemotherapy. In the case of clinical evaluation, the length and width of breast tumors and axillary lymph nodes can be measured with calipers as long as they are palpable. In addition, visual signs of the tumor can be noted, including nipple and inflammatory changes.
Mammography, ultrasound, and MR imaging are typically used in conjunction with one another, although the accuracy of each modality varies in determining extent of disease. DCE-MR shows excellent tissue contrast, but it is an expensive and time-consuming study that requires intravenous contrast administration. Mammography is limited by overlapping tissue and inability to distinguish benign tissue and malignant tissue. Sonography is limited by operator dependence and reproducibility issues, and by posterior acoustic shadowing limiting visualization. Fig. 6 shows the different imaging modalities used in the evaluation of one patient with locally advanced breast cancer. Note that positron emission tomography (PET) is used to evaluate distant disease but may also provide data on local/regional lymph node involvement.