Breast cancer staging and surgical planning are affected by the burden of pathologically proven cancer detected on clinical examination and/or imaging. Magnetic resonance (MR) imaging has superior sensitivity and accuracy for the detection of invasive and in situ breast cancer as compared with physical examination, mammography, and ultrasound but can be limited in specificity. The use of preoperative breast MR imaging for evaluating the extent of disease remains controversial at present because studies have not definitively shown it to improve overall survival, decrease re-excision rates, or to decrease the cost of care.
Key points
- •
Current literature does not support the widespread use of preoperative breast MR imaging.
- •
Patients with lobular cancers are more accurately imaged with MR imaging than with mammography or ultrasound.
- •
MR imaging has been shown to be valuable in assessing the response to neoadjuvant chemotherapy.
- •
All suspicious MR imaging findings should be correlated with biopsy results prior to definitive therapy to ensure appropriate treatment.
Introduction
Magnetic resonance (MR) imaging has demonstrated superior sensitivity for invasive and in situ cancer detection as well as more accurate tumor sizing compared with physical examination, mammography, and ultrasound. Thus, one of the clinical indications for breast MR imaging is for extent of disease assessment in women with newly diagnosed breast cancer. The extent of disease influences the eligibility for breast conservation therapy (BCT), which has been shown to have comparable long-term survival rates with mastectomy but with the benefit of reduced morbidity.
When it comes to extent of disease assessment, the superiority of MR imaging in the detection of otherwise occult breast cancer has been well demonstrated. However, routine use of preoperative MR imaging for patients with newly diagnosed breast cancer has not definitively been shown to improve survival, decrease re-excision rates, or decrease the cost of care. Thus, although the American College of Radiology and the Society of Breast Imaging support the use of preoperative breast MR imaging for clinical management, this remains a controversial indication at present. This article reviews the use of preoperative MR imaging for extent of disease assessment in patients with newly diagnosed breast cancer, including a discussion of specific clinical scenarios and subpopulations.
Introduction
Magnetic resonance (MR) imaging has demonstrated superior sensitivity for invasive and in situ cancer detection as well as more accurate tumor sizing compared with physical examination, mammography, and ultrasound. Thus, one of the clinical indications for breast MR imaging is for extent of disease assessment in women with newly diagnosed breast cancer. The extent of disease influences the eligibility for breast conservation therapy (BCT), which has been shown to have comparable long-term survival rates with mastectomy but with the benefit of reduced morbidity.
When it comes to extent of disease assessment, the superiority of MR imaging in the detection of otherwise occult breast cancer has been well demonstrated. However, routine use of preoperative MR imaging for patients with newly diagnosed breast cancer has not definitively been shown to improve survival, decrease re-excision rates, or decrease the cost of care. Thus, although the American College of Radiology and the Society of Breast Imaging support the use of preoperative breast MR imaging for clinical management, this remains a controversial indication at present. This article reviews the use of preoperative MR imaging for extent of disease assessment in patients with newly diagnosed breast cancer, including a discussion of specific clinical scenarios and subpopulations.
Should MR imaging be used routinely in all patients?
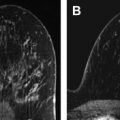
Routine implementation of MR imaging for extent of disease assessment has not been clearly shown to confer a benefit in terms of reoperation rate, disease recurrence, or survival. A meta-analysis of 2600 patients found that preoperative MR imaging detected otherwise occult additional disease in 16% of patients and changed the surgical plan in 11% of cases, which would support the use of routine preoperative MR imaging. However, the Comparative Effectiveness of MRI in Breast Cancer (COMICE) trial from the United Kingdom (with 1600 patients) found a 19% reoperation rate in all patients regardless of whether they underwent preoperative MR imaging. Note that a limitation of the COMICE trial was that not all MR imaging–suspicious findings were biopsied before surgery. As shown in Fig. 1 , not all suspicious MR lesions represent cancer; hence, it is important to prove additional sites of malignancy through biopsy before making a definitive surgical plan (see Fig. 1 ). Another prospective study of 460 patients followed for 8 years found no difference in locoregional recurrence rates or reoperation rates. The Mammography of Nonpalpable Breast Tumors (MONET) trial, which looked at outcomes in more than 400 patients, found that preoperative MR imaging was associated with a paradoxic increase in re-excision rate. This result may be partly attributable to smaller excision volumes in patients with ductal carcinoma in situ (DCIS) and no MR imaging correlate compared with larger excision volumes in patients with DCIS who did not undergo preoperative MR imaging.
With respect to locoregional recurrence rates, the literature has generally not supported widespread use of preoperative MR imaging. One study of more than 300 patients found a significant benefit to preoperative MR imaging in ipsilateral recurrence rates over 3 years (1% vs 7%). However, these data have been criticized for poorly matched MR imaging and control groups. A single-center retrospective study of more than 700 women found no difference in 5-year recurrence rates for women who underwent preoperative MR imaging. This study also found no difference in overall survival rates. The benefit of preoperative MR imaging in reducing locoregional recurrence is likely to remain an unresolved issue for some time because long-term trials of breast MR imaging involving large populations will be required to demonstrate an improvement in the already low recurrence rates (5%–10% at 10 years) for patients with BCT.
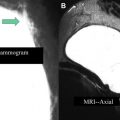
Index tumor sizing is an important factor in appropriate staging. Invasive and in situ tumor sizing by MR imaging correlates better with true pathologic sizing than does mammography or ultrasound ( Fig. 2 ). Mammography and ultrasound were shown to underestimate tumor size by 14% and 18%, respectively, whereas MR imaging tumor sizing did not significantly differ from pathologic sizing.
Preoperative MR imaging has been faulted for increased mastectomy rates, treatment delays, more invasive procedures, additional imaging surveillance, and increased patient anxiety. In MR imaging for extent of disease assessment, biopsy recommendations for the contralateral breast occur approximately 9% of the time, with positive predictive value ranging from 20% to 48%. All MR imaging–suspicious findings should be correlated with tissue sampling to avoid unnecessary surgery.
Given the rapid advances in MR imaging technology with respect to spatial resolution, higher field strength, new coil technology, and the improved capabilities for MR imaging biopsy during the past several years, it is important to continue to assess the utility of breast MR imaging in the preoperative setting. In particular, there are likely specific subpopulations of patients who will benefit from preoperative breast MR imaging.
Invasive cancer with associated extensive intraductal component
The presence of in situ carcinoma surrounding invasive disease (extensive intraductal component [EIC]) is an important prognostic factor, with increased recurrence rates following BCT caused by residual disease. Invasive carcinoma is associated with EIC 30% to 40% of the time. Recurrence rates with BCT for tumors with EIC are lower in the setting of negative margins. Therefore, accurate preoperative assessment of the invasive tumor and surrounding DCIS is mandatory for decreasing reoperation rates.
A recent review by Schouten van der Velden and colleagues on MR imaging of DCIS and EIC reported sensitivities of MR imaging for EIC ranging from 33% to 100%, with most false-negative findings attributable to lower-grade tumors. Gilles and colleagues showed false negatives in DCIS were associated with lesions with weak tumor angiogenesis suggesting the mechanism for the false-negative appearance on MR imaging. Studies have also shown high-grade DCIS to have significantly higher microvessel density than non–high-grade lesions.
Data looking at whether MR imaging appropriately influences surgical planning for EIC tumors is mixed. In the series reported by Berg and colleagues, EIC in 6 of 19 tumors was detected only on MR imaging, with surgical management altered in 5 of the 6 cases of EIC. In another study, MR imaging accurately depicted the extent of disease in all 11 cases of carcinoma with EIC. A study of 23 cases comparing MR imaging and mammographic sizing with histopathologic sizing of tumors with EIC found MR imaging correlated better with histopathologic size than mammography but still overestimated or underestimated the tumor extent 22% and 30% of the time, respectively.
DCIS
Breast MR imaging has demonstrated high detection rates for DCIS compared with the gold standard of mammography (92% for MR imaging vs 56% for mammography). Breast MR imaging is particularly good at detecting high-grade DCIS, with and without comedonecrosis (97%–98%), and reasonably good at detecting low-grade DCIS (80%).
There are 3 primary motivations for the presurgical evaluation of DCIS: accurate estimation of disease extent, identification of occult invasion within the index lesion, and the identification of unsuspected ipsilateral and contralateral foci of disease, both DCIS and invasive cancer. Studies examining the effect of breast MR imaging on DCIS surgical and long-term outcomes are much fewer and smaller in scope than for those for invasive breast cancer and, not surprisingly, have shown conflicting results. Because DCIS is known to have higher re-excision rates than invasive breast cancer, there is a strong motivation to improve the accuracy of preoperative DCIS size estimation. In part, disease estimation in DCIS is hampered by its complex growth pattern, which complicates not only the radiologic but also histopathologic measurement of disease. To date, no imaging modality has been proven to consistently and accurately predict the true extent of DCIS. In a prospective comparison of the 3 modalities in 177 malignant foci, 38 of which were DCIS, Berg and colleagues demonstrated MR imaging to have the highest rate of DCIS visualization (MR imaging, 89%; mammography, 55%; ultrasound, 47%). However, among the 12 patients scheduled for breast conservation, the accuracy of the disease extent was poor when evaluated by any single modality (MR imaging, 42%; mammography, 50%; ultrasound, 33%), with a trend for mammography to underestimate and MR imaging to overestimate disease. Low correlation between MR imaging and pathologic size has been seen in other, albeit retrospective, studies, which vary in their estimation of whether mammography or MR imaging performs more accurately. These studies have not shown preoperative MR imaging to correlate with improved DCIS re-excision rates. Similarly, 8-year rates of local recurrence or survival have thus far been no different between groups that have and have not obtained MR imaging. It may be that DCIS overlaps too much with the appearance of normal tissue to allow an accurate estimation of disease. More likely, the effects of improved MR imaging resolution, together with a better understanding of the imaging appearance and histologic growth pattern of DCIS, will be necessary for breast MR imaging to impact DCIS re-excision and recurrence rates.
Because a core biopsy of pure DCIS can underestimate invasive breast cancer that is ultimately identified at surgery, the question has been raised whether breast MR imaging may preoperatively identify those cases more likely to harbor invasion. The literature broaching this question is sparse. Independent of breast MR imaging, it has been shown previously that the presence of a mass, palpability of the lesion, increased size the index lesion, fewer cores, and high nuclear grade or presence of comedonecrosis correlate with the presence of subsequent invasion found at surgery. Similarly on MR imaging, increased size of the index lesion and the presence of a mass often correlate with the presence of occult invasion, whereas the presence of type I or persistently enhancing kinetics suggests no occult invasion. Although promising, these results as yet have little clinical applicability, given the large overlap in appearance between those cases that do and do not have occult invasion.
The most promising application of breast MR imaging in the preoperative evaluation of DCIS is the evaluation for multicentric and/or contralateral disease. Studies have found breast MR imaging to be useful for identifying additional foci of disease after an initial diagnosis of DCIS. In the study by Hollingsworth and Stough of 285 patients with preoperative MR imaging for DCIS, multicentric or contralateral foci (both in situ and invasive) were found in 5.6% of patients. Distant ipsilateral or contralateral invasive cancer was identified in 3.5% of patients. As Hollingsworth and Stough note, the identification of distant malignancy in the ipsilateral breast or in the contralateral breast requires special consideration for DCIS apart from invasive carcinoma. For any patient diagnosed with DCIS, finding invasive cancer represents an upstaging event. Because the standard treatment of DCIS does not include systemic treatment by endocrine or chemotherapy, contralateral sites will not see any treatment until they eventually come to clinical attention as a second site of disease. As such, preoperative breast MR imaging may prevent these patients from returning years later with a potentially life-threatening second cancer that could have more aptly been treated with the first. Given the high number of false positives by MR imaging, however, it is always recommended that such suspicious sites be sampled first by percutaneous biopsy to avoid unnecessarily wide excision or mastectomy.
Lobular cancers
Lobular cancers tend to grow in thin, wispy single-file strands of cells that are not readily visible on mammography. For lobular cancers, MR imaging detection and sizing accuracy have been shown to be superior to physical examination, mammography, and ultrasound ( Fig. 3 ). With lobular histologies, additional ipsilateral disease is reported approximately 50% of the time. Furthermore, synchronous contralateral disease is reported in 13% of cases.
The data do suggest that preoperative MR imaging for lobular cancers changes surgical management. Change in management based on preoperative MR imaging was reported to occur twice as frequently with lobular cancers than with ductal cancers. In a retrospective study of 267 patients with lobular cancers, the re-excision rate in patients who underwent preoperative MR imaging was one-third of those without preoperative MR imaging. In a recent meta-analysis that included 766 patients with invasive lobular histology, Houssami and colleagues report that MR imaging in lobular cancers may reduce re-excision surgery but at the cost of an increased likelihood of an up-front mastectomy. Given current data, preoperative MR imaging should be seriously considered for patients with lobular histology in the index cancer.
Newly diagnosed cancer in women with dense breasts
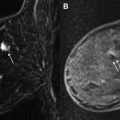
There are limited data regarding MR imaging for extent of disease evaluation in the setting of dense breast tissue. Dense breast tissue limits the sensitivity of mammography because of the masking of cancer by superimposed tissue ( Fig. 4 ). Cancer detection on MR imaging is based on gadolinium enhancement and not affected by breast density. As such, MR imaging would be expected to confer a natural advantage over mammography for evaluating the extent of disease in patients with dense breasts. However, in a prospective, multicenter trial of MR imaging cancer detection in 821 patients, the likelihood of MR imaging–only detected cancer was not significantly related to breast density. In the COMICE randomized trial, breast density was a predefined variable and was not found to significantly alter the rate of re-excision. This unexpected result may relate to the fact that their nondense group consisted only of the mostly fatty breasts, whereas the dense group encompassed everything else, including scattered fibroglandular density.
An Italian study found MR imaging to be more sensitive than mammography in the detection of multifocal disease in dense breasts based on 99 mastectomy specimens. In women with dense breasts who were also at an increased risk of breast cancer, MR imaging detected additional cancers missed by both mammography and ultrasound with a supplemental yield of 14 cancers per 1000 women, as published from the American College of Radiology Imaging Network (ACRIN) 6666 trial. Although ACRIN 6666 was a screening trial, these results demonstrate that MR imaging detects a significant number of additional cancers not seen on mammography or ultrasound in dense-breasted women. Thus, the authors would currently consider the use of preoperative MR imaging to assess the extent of disease in dense breasts, although there is not much literature to support this practice. Fig. 5 presents a sample case of a dense-breasted woman who did not undergo preoperative MR imaging but had positive margins at lumpectomy. Postoperative MR imaging revealed multifocal cancer and a synchronous contralateral cancer prompting the patient to opt for bilateral mastectomies (see Fig. 5 ).