Fig. 14.1
Immunotherapy and vaccination
Among the delivery methods tested, the combination of high frequency ultrasound (1–10 MHz) and gas bubbles (e.g., microbubble, nanobubble, liposomal bubble) was introduced as a physical, non-viral method that is currently under evaluation for gene and drug delivery (See Chaps. 12, 13, 14, 15, 16, 17, 18, and 19). Bubble-assisted ultrasound involves the treatment of a desired volume of cells in-vitro or tissue in-vivo with ultrasound in the presence of bubbles. These bubbles are formulated as lipid, albumin or polymer shelled micrometer (e.g., microbubbles) or nanometer (i.e., nanobubbles, liposomal bubbles) sized gas bodies in aqueous suspension (See Chap. 11). They are then mixed with cells in-vitro or administered by intravascular/intratissue injection in-vivo. The exposure of bubbles to ultrasound causes their periodic oscillations (also referred to as stable cavitation) and/or their collapse (inertial cavitation) under suitable acoustic conditions (See Chap. 9). It is now known that bubble oscillations can induce physical phenomena (e.g., shock waves, micro-streamings, micro-jets) that can disturb the integrity of biological barriers (Doinikov and Bouakaz 2010; Ohl et al. 2006; Ohl and Wolfrum 2003). The use of bubble-assisted ultrasound to deliver therapeutic molecules to diseased tissues has been extensively explored over the past decade. Indeed, this technology has been successfully used to transfer nucleic acids into the heart, vessels, liver, skeletal muscle and tumors (Escoffre et al. 2013b). In addition, in-vivo local delivery of low molecular weight chemotherapeutics has been reported (Escoffre et al. 2013a; Iwanaga et al. 2007) and is now under clinical investigation (Kotopoulis et al. 2013). For both in-vitro and in-vivo applications, this technology enables delivery of exogenous molecules with minimal cell or tissue damage, inflammation and/or immunological response (Hauff et al. 2005; Taylor et al. 2007; Escoffre et al. 2010a). In addition, ultrasound can be non-invasively targeted to a specific volume of superficial tissue (e.g., skin), as well as deeply embedded organs (e.g., liver). Together, these properties make bubble-assisted ultrasound an innovative and compelling method for the delivery of nucleic acids, antibodies (Abs), cytokines and Ags.
Recently, the applications of bubble-assisted ultrasound have been extended to immunotherapy and vaccination, especially for the treatment of brain diseases and cancer. The focus of this chapter is to review the current applications of bubble-assisted ultrasound in these fields. The limitations and the future developments of bubble-assisted ultrasound will be further discussed.
14.2 Ab-Based Immunotherapy
14.2.1 Applications in Neurology
The increasing knowledge of molecular mechanisms of brain diseases and the absence of successful therapies allowed the advent of Ab-based immunotherapies for the treatment of neurodegenerative diseases. Nevertheless, this attractive therapeutic option is restricted by a physiological barrier, the blood-brain barrier (BBB) (Wong et al. 2013). This barrier plays a major role in the brain homeostasis. The BBB consists of pericytes, astrocytes, neurons and microglia and endothelial cells in close contact. The endothelial cells of the central nervous system (CNS) are unique cells that are tightly fused to each other by intercellular attachments known as tight junctions. The BBB limits the passage of most molecules (e.g., nucleic acids or antibodies) from the circulation into the brain parenchyma, thus precluding their use in the treatment of neurodegenerative diseases and brain tumors. The factors that determine molecular extravasation are their molecular charge and size (e.g., 150 kDa for Abs), their lipid solubility (e.g., Abs are hydrophilic) and whether or not these molecules use any transport processes across the BBB (e.g., transcytosis).
To overcome these limitations, different strategies of antibody delivery across the intact BBB have been evaluated. These include chemical or genetic modification of the antibodies with cationic compounds (i.e., absorptive-mediated transcytosis) or with endogenous molecules necessary for normal brain function (i.e., receptor-mediated transcytosis) (Chacko et al. 2013). However, the main limitation of these approaches is the putative loss of Ab function. Transient BBB disruption (BBBD) is another strategy that improves intracerebral delivery of antibodies. Indeed, intra-arterial (i.a.) injection of hyperosmotic solutions (e.g., 1.6 M mannitol) or pharmacological agents (e.g., bradykinin) has been used to induce a transient BBBD, which was, however, non-focal and involved the entire brain tissue supplied by the injected artery branch. Local BBBD can be clinically performed by direct stereotaxis-guided injection of molecules into the targeted brain site, however this method remains invasive.
Nowadays, many efforts are being made to increase the BBB permeability to facilitate local delivery of therapeutics into the brain. Since the 1990s, local BBBD using microbubble-assisted ultrasound under magnetic resonance imaging (MRI) guidance is under investigation (Aryal et al. 2014; Burgess and Hynynen 2014). Thus, ultrasound-driven microbubble oscillations near the BBB may promote the extravasation and the uptake of antibodies into the desired brain region. MRI provides the identification of the target location, and allows the assessment of the efficacy of the BBBD on the basis of the extravasation of MRI contrast agents. Owing to this method, several groups around the world have successfully reported the targeted delivery of chemotherapeutics, genes, therapeutic Abs and the extravasation of immune or stem cells into the CNS (See Chap. 16).
Example – Alzheimer’s disease
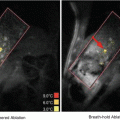
Alzheimer’s disease (AD) is a progressive and incurable neurodegenerative disorder that affects several tens of millions of people worldwide. AD pathogenesis is thought to be correlated to the accumulation of beta-amyloid (Aβ) plaques and hyperphosphorylated tau tangles (Ittner and Gotz 2011). New knowledge of the pathogenesis of AD has allowed emerging diagnostic and therapeutic strategies that aim at detecting and reducing Aβ plaques. Ab-based immunotherapy is a promising therapeutic option for Aβ plaque clearance, either by active immunization with Aβ peptides or genes, or by passive immunization after administration of anti-Aβ Abs (Liu et al. 2012). Current treatment of AD patients requires long-term intravenous (i.v.) perfusion of high doses of anti-Aβ Abs in order to remove Aβ plaques from the brain (Opar 2008; Wilcock and Colton 2008). In AD mouse models, i.v. and i.a. injection of a high dose of 500 μg anti-Aβ Abs resulted in clearance of Aβ plaques and cognitive progress, while pharmacokinetic analyses revealed that less than 1 % of Abs actually reached the brain (Bard et al. 2000; DeMattos et al. 2001; Banks et al. 2002). However, Thakker et al.., reported that the intracerebral injection of 40 μg anti-Aβ Abs was more efficient at clearing Aβ plaques than intra-peritoneal (i.p.) administration of 200 μg Abs (Thakker et al. 2009). Hence, targeted intracerebral delivery of anti-Aβ Abs allows an effective amount of Ab access to the Aβ plaque-heavy anatomical regions, thus leading to an improved treatment in AD mice models. However, most technical procedures required to achieve this are invasive. In this context, Raymond et al. investigated anti-Aβ Ab delivery into the brain of AD mouse models using MRI-guided and microbubble-assisted focused ultrasound (MRIgFUS) (Raymond et al. 2008). MRI guidance allowed selective treatment and monitoring of affected brain regions (Fig. 14.2a, b).


Fig. 14.2
Anti-Aβ Abs (BAM-10) delivery using microbubble-assisted FUS under MRI guidance (MRIgFUS). Mice were positioned in a supine position (a). The right sides of mice brains were targeted with FUS after i.v. co-injection of Definity®, Gadovist® and anti-Aβ Abs (BAM-10). T1-weighted contrast-enhanced MRI scans were used to position ultrasound foci prior to (b) and following (b′) transcranial FUS treatment. BAM-10 antibodies (green stain) and Aβ plaques (red stain) were detected using immunofluorescence assays at 4 h (c, c′), 2 days (d, d′) and 4 days (e, e′) post-treatment (scale bar 50 μm). In 4 days, the mean count (f), size (g) and surface area (h) of Aβ plaques of right sonicated and left unsonicated sides of mice brains were determined (Reprinted with permission from Jordao et al. (2010))
After i.v. injection of anti-Aβ Abs (0.1 mL of 53 mg/mL whole serum), mice were sonicated (0.69 MHz, PRF1 of 1 Hz, DC2 of 1 %, PNP3 of 0.67–0.8 MPa, duration of 40–45 s) with concomitant i.v. administration of Definity® microbubbles (0.01 mL 1:10 diluted contrast agent). Subsequently, BBB disruption was monitored with T1-weighted fast spin echo MRI following i.v. injection of Magnevist® (MR contrast agent; 62.5 μL/kg). The results revealed that sonicated brain regions displayed a 2.7 ± 1.2-fold increase in anti-Aβ Abs binding to Aβ plaques. No Ab extravasation was detected in the unsonicated regions. In addition, T1-weighted fast-spin echo MRI and anti-Aβ Abs detection in sonicated regions showed a positive correlation between the MRI intensity change and Ab concentration, thus indicating that the BBBD was responsible for the intracerebral delivery of anti-Aβ Abs. Afterwards, this procedure was successfully reproduced in two different transgenic strains (i.e., APPswe:PSEN1dE9, PDAPP) across a large age range (i.e., 9–26 months). Little or no tissue damage (i.e., scattered petechia) was detected in sonicated brain tissues, thus revealing the safety of this approach.
This same group then continued to investigate the therapeutic benefit of anti-Aβ Abs with FUS-mediated BBBD in a transgenic AD mouse model (i.e., TgCRND8) (Jordao et al. 2010). Following a similar procedure, the mice brains were targeted with ultrasound (0.558 MHz, 10 ms burst/Hz, PNP of 0.3 MPa, duration of 120 s) after i.v. co-administration of Definity® (160 μL/kg), Gadovist® (MR contrast agents; 0.1 mL/kg) and 40 μg of anti-Aβ Abs (BAM-10) (Fig. 14.2a). Within minutes following the sonication, Gadovist® was taken up in the targeted brain tissues (Fig. 14.2b) and anti-Aβ Abs were later detected bound to Aβ plaques (Fig. 14.2c–e). Four days post-treatment, the number and mean size of Aβ plaques as well as surface area of Aβ were significantly decreased in right sonicated side of mice brain compared to left unsonicated side of brain (Fig. 14.2f–h). In conclusion, microbubble-assisted FUS under MRI guidance is a promising non-invasive technology for the local delivery of low but therapeutic doses of anti-Aβ Abs.
14.2.2 Applications in Oncology
Cancer therapy has considerably developed over the past decade, especially in the area of therapeutic Abs (Scott et al. 2012). Unlike chemotherapy, Abs are able to distinguish between healthy and tumor tissues, thus potentially providing efficient therapy, while minimizing adverse side effects to healthy tissues. Abs potentially provide effective therapy by targeting specific molecular targets involved in biological pathways of tumor growth and dissemination, thus leading to tumor cell growth inhibition. In in-vivo experiments, Abs activity is found to depend on natural killer cell action, identifying antibody-dependent cellular cytotoxicity (ADCC) as the main mechanism of Abs action. Recently, the FDA and EMA have approved several Ab-based immunotherapies for the clinical treatment of hematological malignancies (e.g., Zevalin®, Rituxan®) and solid tumors (e.g., Erbitux®, Herceptin®). In clinical trials, these Abs decreased mortality in patients with metastatic or early cancer, either as a monotherapy or combined with chemotherapy.
However, Ab-based immunotherapy is rarely curative in solid tumor. Owing to their poor intratumoral (i.t.) bioavailability, systemic injection of high or frequent Ab doses is necessary to reach therapeutic efficacy. Consequently, these Ab doses are associated with severe side effects and this immunotherapy becomes an expensive option. Indeed, the limited success of this approach for solid tumors is due to a number of factors, including Ab physicochemical properties, and the biological characteristics of the tumor microenvironment (Frenkel 2008). The large size of antibodies (150 kDa) may provide a long circulatory half-life (t1/2 > 21 days in humans), but on the other hand decreases its extravasation and penetration into tumor tissues. Contrary to healthy tissues, tumor tissues have a high interstitial fluid pressure due to their leaky vasculature and lack of functional lymphatics (Boucher et al. 1990). These high pressures establish an outward fluid motion from the periphery of the solid tumor and reduce fluid infiltration across the vascular wall. As a result, the extravasation and tumor accumulation of convection-dependent macromolecules, such as Abs, are severely limited. The increase in mean distance between tumor cells and vessels is another limitation for insufficient delivery of Abs. Indeed, high cell proliferation levels lead to tumor cells forcing vessels apart, thus resulting in a decrease in vascular density and a limitation in the access of Abs to distant tumor cells (Minchinton and Tannock 2006). Once in the tumor interstitium, extracellular matrix and binding-site barriers limit the interstitial transport of Abs through non-specific and specific interactions, respectively. Altogether these limitations prevent sufficient and uniform distribution of Abs in solid tumors. Recently, microbubble-assisted ultrasound has been investigated to improve Ab delivery in oncology.
Example 1: Cetuximab and Head and Neck Squamous Cell Carcinoma
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide with an estimated global incidence of 500,000 new cases and is known for its rapid clinical progression. During the past 25 years, treatments combining surgery, radiotherapy and chemotherapy have improved disease outcome, but this is linked to significant patient morbidity and an increase in treatment-related deaths (Price and Cohen 2012). The increasing knowledge on HNSCC pathogenesis allowed the identification of a new molecular target, the epidermal growth factor receptor (EGFR) (Bose et al. 2013). EGFR was found to be overexpressed in HNSCC and has been associated with more advanced disease and unfavorable outcomes. These investigations led to the development of a novel targeted therapeutic, cetuximab (Erbitux®), a chimeric human:murine IgG1 monoclonal antibody that targets EGFR. Clinical trials have revealed modest outcome improvements in HNSCC treated with this Ab, especially when combined with radiotherapy (Bonner et al. 2006). In addition, adverse side effects of cetuximab treatment alone or combined with other therapies still significantly contribute to patient morbidity (Logan 2009). In this context, any method increasing in intra-tumor cetuximab bioavailability, while simultaneously minimizing side effects to healthy tissues, would be an attractive method for HNSCC therapy.
Recently, Heath et al. examined the therapeutic benefit of cetuximab delivery by microbubble-assisted ultrasound for the treatment of HNSCC (Heath et al. 2012). In-vitro results showed that this method (Definity® microbubbles; 1 MHz, MI4 of 0.5, PRP5 of 0.01 s, DC of 20 %, duration of 5 min) resulted in a 30 % increase in intracellular uptake of cetuximab in HNSCC cells compared to cetuximab treatment alone. In agreement with previous data, this enhanced Ab incorporation was positively correlated to a significant increase in cell apoptosis. In-vivo cetuximab delivery using microbubble-assisted ultrasound (Definity® microbubbles; 1 MHz, MI of 0.5, PRP of 5 s, DC of 20 %, duration of 5 min) in subcutaneous HNSCC tumor xenografts led to a 30 % decrease in tumor size compared to cetuximab treatment alone. Histopathological analyses of tumors revealed that the reduction in tumor size was positively correlated to a decrease in microvessel density and to an increase in apoptosis. In addition, no tissue damage was reported in the tumor surrounding tissues after treatment. In conclusion, cetuximab delivery using microbubble-assisted ultrasound showed a therapeutic benefit.
Example 2 – Trastuzumab and Brain Metastatic Breast Cancer
Breast cancer is the most common cancer in women worldwide and the principle cause of death from cancer among women. Moreover, 25–30 % of human breast cancers overexpress the human epidermal growth factor receptor (HER-2), which is predictive of poor clinical outcome (Arteaga et al. 2012). HER-2 positive breast cancer has been found to metastasize to the CNS more frequently than all other breast cancer types (Leyland-Jones 2009). The prognosis for patients with CNS metastases is in majority very poor. The standard treatments are steroids combined with radiotherapy, surgery or stereotaxic radiosurgery (Chang and Lo 2003). As previously described, chemotherapy is generally excluded as an effective therapeutic option due to the BBB. However, in most primary brain tumors and metastases, the permeability of the tumor vasculature is heterogeneous, thus facilitating free access of toxic concentrations of chemotherapeutics to a small subset of highly perfused metastases (Eichler et al. 2011). Nevertheless, metastatic seeds could be protected by the BBB of the surrounding healthy tissues. Thus, the BBB remains one of the main hindrances to patient cure. Unfortunately today, facing the lack of efficient therapeutic alternatives, the number of women with CNS metastases and recurrence after radiotherapy is still increasing.
Trastuzumab (Herceptin®) is a humanized monoclonal Ab that targets HER-2 overexpressed in breast tumors (Hudis 2007). This immunotherapy decreases mortality in patients with early and metastatic breast cancer, either as a monotherapy or combined with chemotherapy and/or radiotherapy (Joensuu et al. 2006; Horton et al. 2010). This survival benefit is mostly due to control of systemic disease, with the CNS remaining a sanctuary site. Nevertheless, clinical investigations have reported that the increasing use of trastuzumab has resulted in a higher incidence of brain metastases from primary tumors (Bendell et al. 2003).
In order to effectively treat CNS metastases, trastuzumab will need to overcome the limitations imposed by the BBB and the tumor microenvironment. Recently, Kinoshita et al. investigated the use of microbubble-assisted ultrasound to deliver trastuzumab into the brain of healthy mice (Kinoshita et al. 2006). Using a 0.69 MHz focused ultrasound transducer and the i.v. co-injection of trastuzumab (20 mg/kg) and Optison® microbubbles (1.6 mL/kg) with Magnevist® (1.6 mL/kg), the BBB was non-invasively opened under MRI guidance. After 0.6 MPa or 0.8 MPa sonication (PRF of 1 Hz, DC of 1 %, duration of 40s), the amount of trastuzumab in the targeted brain tissue increased to 1.50 and 3.25 ng/g of tissue, respectively. No trastuzumab was detected in unsonicated brain tissues. This intracerebral delivery was positively correlated to BBBD monitored by T1-weighted fast-spin echo MRI. In addition, histopathological analyses revealed that the sonication resulted in few scattered, extravasated red blood cells and apoptotic cells, whose the numbers increased with increasing acoustic pressure. However, no ischemic neurons were detected in the sonicated brain tissue. In conclusion, this technology is a promising method to increase the intracerebral bioavailability of trastuzumab.
Six years later, Park et al. assessed the therapeutic benefit of this method in a rat model of breast cancer metastases in the brain (Park et al. 2012). To this end, HER-2 positive human breast cancer cells (i.e., BT-474) were intracranially implanted into nude rats. Animals received 6 weekly sonication treatments (0.69 MHz, PRF of 1 Hz, DC of 1 %, PNP of 0.69 MPa, duration of 60s) under MRI guidance, combined with i.v. injection of trastuzumab (2 mg/kg), Magnevist® (0.2 mL/kg) and Definity® microbubbles (10 μL/kg) (Fig. 14.3). Tumor growth and survival rates were monitored using MRI for 7 weeks after treatment. Trastuzumab delivery using microbubble-assisted ultrasound resulted in a significant decrease in tumor volume compared to Ab treatment alone (Fig. 14.3a, b). At the study endpoint, tumor eradication was only observed in rats treated by microbubble-assisted ultrasound combined with trastuzumab administration. More than half treated rats survived, resulting in a median survival time of more than 83 days (i.e., at least 17 % longer than trastuzumab treatment alone). To conclude, trastuzumab delivery using microbubble-assisted ultrasound showed a significant therapeutic benefit compared to trastuzumab treatment alone.


Fig. 14.3
Trastuzumab delivery using microbubble-assisted FUS under MRI guidance (MRgFUS). Serial T1-weighted images (T1WI) were acquired before (Pre-FUS) and after (Post-FUS) six treatments with trastuzumab delivered by microbubble-assisted FUS. Human HER-2 positive breast tumor disappeared after 6 weeks (a) and another one did not exhibit a strong response (b). Post-treatment images were acquired after Magnevist® injection. Yellow contours show the brain regions where the signal intensity was at least 5 % above that in non-sonicated brain regions (Reprinted with permission from Park et al. (2012))
14.3 Dendritic Cell-Based Vaccination
Dendritic cells (DCs) are the most potent antigen presenting cells (APCs), and they are able to prime and activate cytotoxic T-lymphocytes (CTL or CD8+ T cells) and T helper lymphocytes (Th or CD4+ T cells) responses (Abbas and Lichtman 2006). CD8+ T cells are involved in the elimination of infected or transformed cells, whereas CD4+ T cells essentially support efficient and long-lasting T and B lymphocyte responses through cytokine production and intercellular interactions. Free Ags induce a potent CD4+ T cell response, while the induction of CD8+ T cell responses is efficiently achieved as a result of the cross-presentation of particle-coupled Ags. In addition, DCs are also involved in the generation of Ab responses through their interactions with B lymphocytes. Immature DCs function as sentinel cells throughout the organism periphery where they recognize and engulf Ags. These Ags are subsequently processed, and DCs migrate to afferent/draining lymph nodes (i.e., secondary lymphoid organs) where the local initiation of immune responses occurs. DCs mature into APCs during their migration, and these mature DCs present epitope peptides derived from Ags via major histocompatibility complex (MHC) class I molecules and MHC class II molecules. At the same time, exogenous Ags may have direct access to the draining lymph node where they are recognized by resident DCs, and a suitable profile of adaptive immune responses is generated under the control of environment-mediated signals. While innocuous Ags usually activate tolerogenic cells, Ags associated with danger signals, such as harmful pathogens, ultimately trigger effector immune cells.
Currently, immunization techniques using live attenuated and killed pathogen-based vaccines have proven efficient immune response induction, thus controlling various infections (Andre 2003). However, such vaccines are not useful for the prevention or the treatment of cancer, autoimmune and allergic disorders. Besides, the use of live attenuated pathogens for vaccination raises extensive safety issues. To overcome these limitations, subunit vaccines based on recombinant or purified Ags have emerged as an alternative option (Foged 2011). Nevertheless, these Ags are poorly immunogenic. Addition of immune-modulating molecules/adjuvants has successfully been described to increase the immunogenicity of recombinant Ag-based vaccines. For decades, research has focused on the development of efficient and safe methods to deliver Ags, peptides, mRNA and pDNA into DCs (Cintolo et al. 2012). These methods include the absorption or covalent linkage of Ags to particles (e.g., lipid or polymer-based nano- or micro-particles), mineral salts (e.g., alum) and antibodies, as well as their encapsulation inside particles. The efficiency of these methods relies on their ability to prevent Ag degradation, thus delivering effective concentrations of Ags into DCs to activate them. The combination of efficient Ag delivery methods with immuno-stimulating molecules could be crucial for the induction of efficient and long-lasting effector and memory immunities. Recently, several studies reported bubble-assisted ultrasound as a promising and efficient method to deliver Ags into DCs (Lemmon et al. 2011; Un et al. 2010, 2011; Oda et al. 2012; De Temmerman et al. 2011). Indeed, recombinant Ags, peptides or nucleic acids can be loaded on gas bubbles during their assembly. Besides, bubbles provide additional protection against Ag degradation. In the following examples, the combination of these intelligent carriers with ultrasound has been successfully reported for the delivery of Ags into DCs for the treatment of melanoma lung metastasis.
Example 1: Delivery of Melanoma-derived Ag Proteins
Melanoma is the leading cause of skin cancer death worldwide and has early tendency to metastasize (Black and Brockway-Lunardi 2013; Ferlay et al. 2013). The 5-year survival of patients with localized melanoma (i.e., confined to the primary site) is between 80 and 90 %, while patients with metastasized melanoma have a 5-year survival below 20 %. The chemo-resistance of melanoma, along with the highly tumor heterogeneous response rate for any single or combination of chemotherapeutics, explains that metastatic melanoma leads to the majority of melanoma deaths. As a result, the development of effective therapeutic options, including DC-based immunotherapy, is becoming a challenge for the treatment of metastasized melanoma (Sanlorenzo et al. 2014).
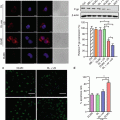
Recently, prophylactic immunization with DCs, exposed to Ags during bubble liposome (BL)-assisted ultrasound, was investigated for preventing melanoma lung metastasis (Oda et al. 2012). In this approach, the Ags were extracted from melanoma cells and were delivered (50 μg) into DCs using BL-assisted ultrasound (2 MHz, DC of 10 %, burst rate 2 Hz, 2 W/cm2, 3 × 10s with time interval of 10 s; 120 μg of BLs) (Fig. 14.4).


Fig. 14.4
Prophylactic immunization with BLs and ultrasound-treated dendritic cells (Reprinted with permission from Oda et al. (2012))
The delivery efficiency was approximately 74 %. Unlike Ag delivery methods previously described, relying on endocytotic pathways; BL-assisted ultrasound directly delivers the melanoma-associated Ags (MAA) into the cytosol. Accordingly, this approach induces MHC class I-restricted MAA presentation on DCs. To assess the in-vivo prophylactic immunization, C57BL/6 mice were immunized twice with DCs before i.v. injection of B16/BL6 melanoma cells. Histopathological analyses revealed that DC-based immunotherapy provided a fourfold decrease in the frequency of melanoma lung metastasis. This investigation demonstrated that BL-assisted ultrasound is an encouraging method for Ag protein delivery into DCs (Fig. 14.5).