Calcifications
Calcifications developing in the breast are variable in number and appearance. Most calcifications detected in patients on screening mammograms reflect a benign etiology. A small percentage develop in association with ductal carcinoma in situ (DCIS) or, less commonly, the invasive component of ductal carcinomas. It is incumbent on the radiologist to detect, evaluate, classify, and make appropriate recommendations for calcifications perceived on mammograms.
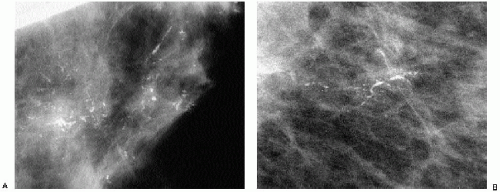
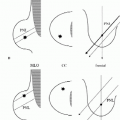
Complete mammographic workups (Chapter 3), coupled with a knowledge of breast anatomy, histology, and histopathology, are critical in understanding the mammographic appearance of breast calcifications and establishing appropriate and justifiable recommendations. Calcifications developing in a space (i.e., ducts or acini) are molded by that space. Calcifications arising in ducts are tubelike or linear and may demonstrate linear distribution. If they develop in subsegmental ducts, they are large, rodlike, and dense compared with the smaller calcifications of variable density that develop in terminal ducts. When the epithelial lining is attenuated or denuded, the borders of the calcifications are smooth (Figure 5.1) compared with the irregular margins that may be seen when there is active cellular proliferation and necrotic debris in the lumen of the duct (Figure 5.2). Calcifications forming in acini are round or punctate (Figure 5.3). If the normally round acini are compressed, elongated, or deformed by proliferation of the surrounding perilobular stroma, the calcifications may demonstrate pleomorphism, including round, punctate, oval, and comma-shaped forms.
As discussed in Chapter 2, establishing optimal film quality is one of the initial steps in reviewing screening and diagnostic mammograms. Well-exposed, high-contrast images with optimal positioning and no blurring are essential in maximizing our ability to detect microcalcifications and small spiculated masses. The acceptance and interpretation of suboptimal films can result in a delay in the diagnosis of breast cancer.
Image quality is particularly critical in our ability to detect (screening) and characterize (magnification views) microcalcifications.
When evaluating calcifications, consider the following characteristics: What is the form of the calcifications? Is it round or linear, coarse or fine (granular), monomorphic or pleomorphic within a cluster? What is the size of the calcifications? Is it large or small, and if in a cluster, are the individual calcifications homogeneous in size? What is the density of the calcifications? Is the density high or low? In a given cluster, is there homogeneity in density among the individual calcifications? What is the distribution of the calcifications? Is the distribution unilateral or bilateral, single cluster or multifocal, diffuse, regional, segmental, or linear? Diffuse bilateral calcifications, scattered in dense tissue, are typically benign, in contrast to linear calcifications in a segmental distribution.
Although some emphasis has been placed previously on the number of calcifications in a given cluster, we do not find this to be a particularly helpful characteristic. One or two linear calcifications with irregular margins may be related to the presence of DCIS and
require a biopsy. Conversely, a tight cluster of many round, pearl-like calcifications of homogeneous density do not typically require a biopsy.
require a biopsy. Conversely, a tight cluster of many round, pearl-like calcifications of homogeneous density do not typically require a biopsy.
In evaluating a cluster of calcifications ask yourself: are there linear forms with irregular borders, or is there a linear distribution of round, oval, punctate calcifications?
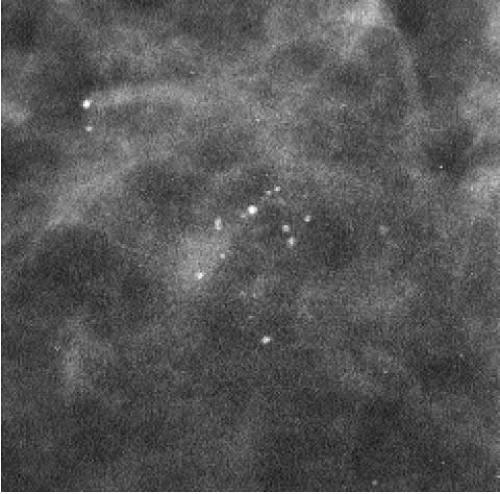
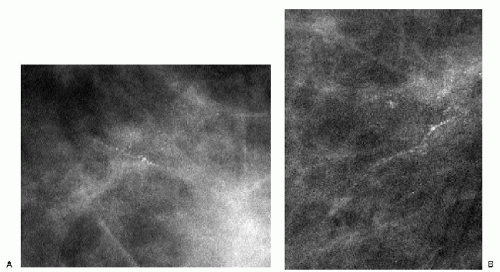
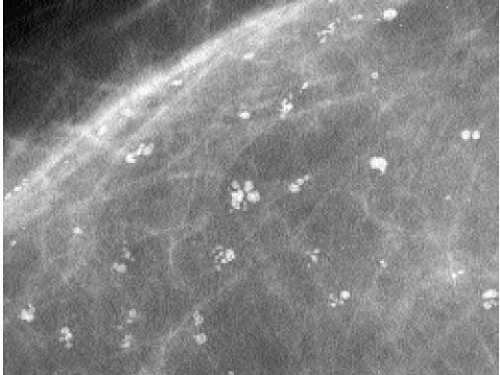
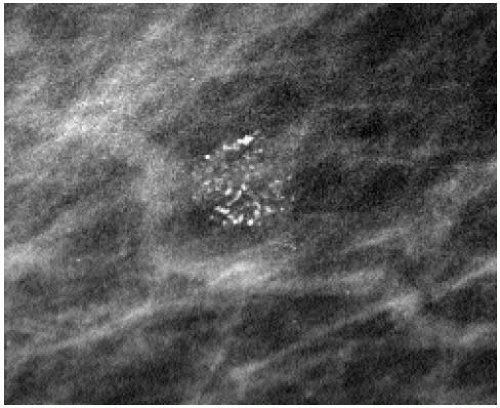
The presence of linear calcifications with irregular borders, variable density, and linear orientation that are haphazardly or segmentally distributed needs to be considered significant (Figure 5.4). The likelihood of an underlying DCIS (low, intermediate, or high nuclear grade) associated with central necrosis, is high; biopsy is indicated in these patients. These types of calcifications are rarely associated with benign processes including those listed in Box 5.1. We also consider the linear distribution of round, oval, punctate, or amorphous calcifications, with variable density, an indication for biopsy (Figure 5.5). However, in the absence of linear calcifications or a linear distribution, the likelihood of DCIS drops significantly and, when diagnosed, is usually, although not exclusively, lowor intermediate-grade DCIS with no central necrosis. Benign diagnostic considerations for clusters composed of round, oval, punctate (Figure 5.6), or amorphous calcifications include those listed in Box 5.2.
Box 5.1: Linear, Casting, Branching, and Pleomorphic Calcifications
Ductal carcinoma in situ (with central necrosis; commonly high nuclear grade but can also be seen in about 20% of low- or intermediate-grade cases)
Fat necrosis (in the early stages of calcification)—rare
Fibroadenoma—rare
Dystrophic, fibrosis—rare
Autoimmune disorders (dermatomyositis, scleroderma, lupus)—rare
Box 5.2: Cluster of Punctate, Round Calcifications
Lobular calcifications
Sclerosing adenosis
Fibroadenoma
Fibrocystic change (hyperplasia, atypical ductal hyperplasia)
Papilloma
Ductal carcinoma in situ (more commonly low or intermediate cribriform, micropapillary cases without central necrosis)
BENIGN BREAST CALCIFICATIONS
For didactic purposes, I take an anatomic approach in describing breast calcifications. However, the terms provided in the American College of Radiology (ACR) BIRADS lexicon (1) are presented in Table 5.1 and Box 5.3 and used throughout the text.
As mentioned previously, when thinking about breast calcifications, consider the anatomic structures available for breast calcifications to develop and the potential pathologic processes associated with these structures. This becomes helpful when analyzing calcifications and determining appropriate diagnostic considerations and management. Include the following tissue types: skin, fibrous stroma, ducts (large and small), acini grouped into lobules and arteries. Calcifications developing in masses may be associated with the wall (mural) of the mass, if one is present (e.g., cysts, oil cysts), or with the epithelial or stromal elements of the mass. If the mass contains fluid, the calcifications may be in suspension (e.g., milk of calcium). Rarely, calcifications develop in association with foreign bodies, such as suture material and parasites.
In thinking about breast calcifications, consider the anatomic structures available in breast tissue within which calcifications can develop and the potential (pathologic) processes involving these structures.
Table 5.1: American College of Radiology Lexicon, Descriptive Terms for Calcifications | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Box 5.3: American College of Radiology Lexicon, Distribution Modifiers for Calcifications
Group or clustered (neutral terms)
Linear
Segmental
Regional
Diffuse, scattered
SKIN (DERMAL) CALCIFICATIONS
Skin calcifications (Figure 5.7) form in dermal sweat glands after low-grade folliculitis and inspissation of sebaceous material. Consequently, they are:
Radiolucent-centered calcifications are benign.
Round or oval
Lucent centered
Isolated, or more commonly, multiple clusters bilaterally
Lacelike pattern when associated with moles
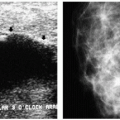
Commonly, skin calcifications are seen posteromedially projecting on the pectoral muscle on the mediolateral oblique (MLO) view and medially, at the cleavage, on craniocaudal (CC) views; they can involve the skin diffusely.
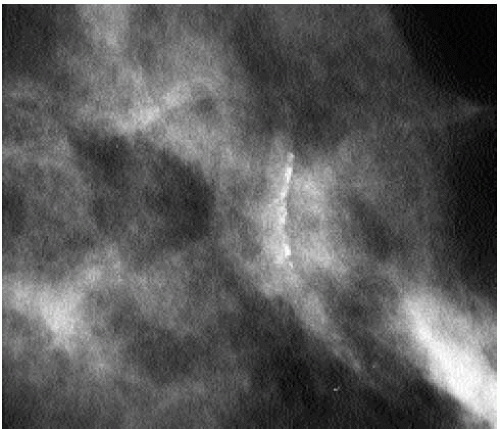
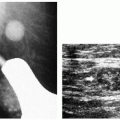
On any two views, much of the skin projects superimposed on the breast parenchyma; only a small amount of skin is ever tangent to the x-ray beam, enabling distinction between skin and associated lesions from underlying breast tissue. Although the appearance of most skin calcifications is distinctive, if lucent centers are not readily apparent, definitive diagnosis is made when the skin surface containing the calcifications is imaged tangent (Figure 5.8) to the x-ray beam (2, 3, 4) (Chapter 3).
In some patients, calcifications develop in association with moles or other skin lesions (e.g., sebaceous cysts). These can outline the crevices of the mole, creating semicircular, lacelike calcifications (Figure 5.9); or, in some patients, they may be pleomorphic (Figure 5.10). In some women, talc, zinc oxide ointment (Desitin), or other high-density products deposited in the crevices of moles, can simulate calcifications. The overall density of these particles, their morphology and distribution are usually pathognomonic (Figure 5.11); alternatively, either placing a metallic BB on the skin lesion and demonstrating that the BB moves with the lesion on two views or obtaining a tangential view of the skin lesion provides definitive diagnosis.
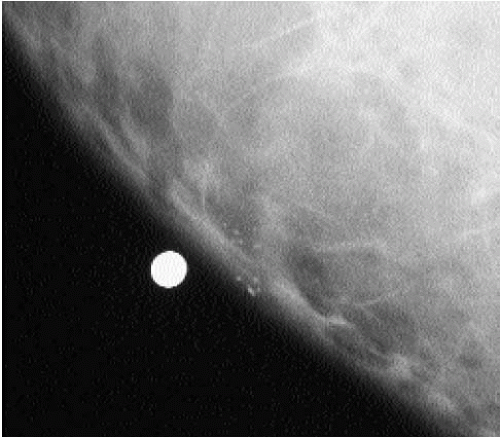 Figure 5.8 Skin calcifications. Spot tangential view demonstrating the dermal location of a cluster of round and oval calcifications, lacking a lucent center. Category 2. |
VASCULAR CALCIFICATIONS
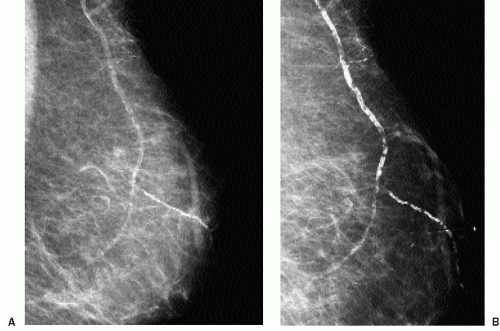
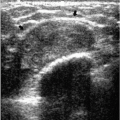
Deposition of calcium in the media, at the perimeter of the elastic fibers of arterial walls, results in dense, linear, parallel, or tram-track-like calcifications, most commonly in postmenopausal women with arteriosclerotic heart disease (Figure 5.12). When seen in younger, premenopausal women, it is often in patients with diabetes. When only a portion of the arterial wall is affected, or when small vessels are involved, the calcifications can simulate those occurring in DCIS because at this stage, the calcifications may be linear, irregular, variable in density, and have a linear orientation (Figure 5.13). In these patients spot compression magnification views demonstrate the contralateral uncalcified vessel
wall, the uncalcified portion of the vessel coming into and going out of the area of the calcifications, or an accompanying vein (Figure 5.14).
wall, the uncalcified portion of the vessel coming into and going out of the area of the calcifications, or an accompanying vein (Figure 5.14).
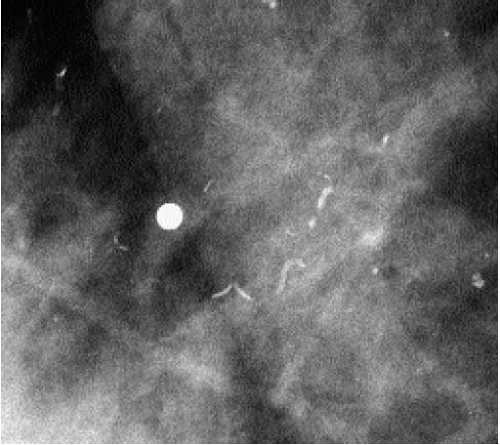
Occasionally, smaller vessels calcify, leading to the appearance of “quirky” types of calcifications. The borders of these calcifications are well defined, and a lucent center is often seen when evaluated closely with a magnifying lens (Figure 5.15). In our experience, these smaller arterial calcifications tend to be more common in premenopausal women with
dense tissue and can fluctuate from year to year; in some patients, they resolve completely. These patients do not usually have a history of diabetes or arteriosclerotic heart disease.
dense tissue and can fluctuate from year to year; in some patients, they resolve completely. These patients do not usually have a history of diabetes or arteriosclerotic heart disease.
It has been reported by several investigators that there may be a correlation between the extent of arterial calcifications seen on a mammogram and underlying coronary artery disease. The presence of extensive arterial calcifications should be described in mammographic reports (5, 6, 7). Rarely, tortuous and serpiginous calcifications associated with a venous structure, may be seen as a long-term sequela of Mondor’s disease (8).
Consider describing the presence of extensive, bilateral vascular calcification in the mammography report.
DYSTROPHIC CALCIFICATIONS
Dystrophic calcifications form in stromal fibrous tissue, not in predefined anatomic spaces; consequently, they vary in size, shape, and density; no two of these calcifications are alike. They are coarse, dense, large, and irregularly shaped and may have associated areas of lucency (Figure 5.16). These are associated with benign conditions, such as fat necrosis related to prior trauma (Chapter 6), burns or surgery, radiation therapy (Chapter 9), a healed abscess, or hematoma. When diffuse and bilateral, they may reflect the presence of an underlying inflammatory, degenerative, or metabolic process (e.g., renal disease, hyperparathyroidism). Dystrophic calcifications can also be seen in the fibrous capsule that forms around implants or in conjunction with the granulomatous response elicited by foreign bodies, such as silicone or paraffin injections.
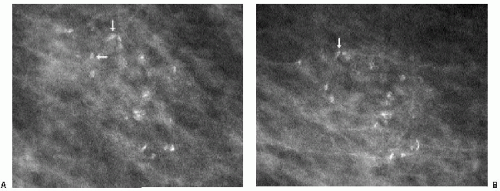
Some dystrophic calcifications demonstrate a linear appearance (Figure 5.17). These are commonly found in areas of dense stromal fibrosis (Figure 5.18). The margins are irregular and jagged, with some of the calcifications forming acute angles. In a given cluster, some of the linear calcifications are well defined with central lucencies (Figure 5.19).
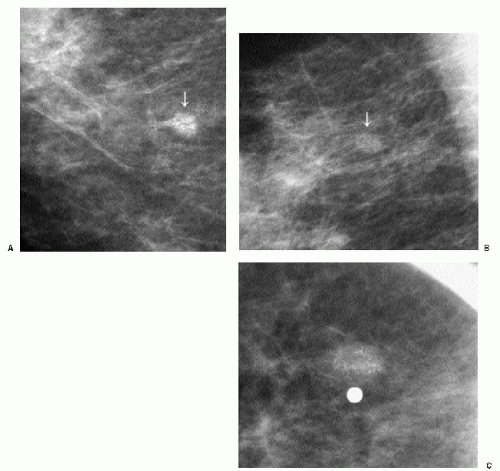
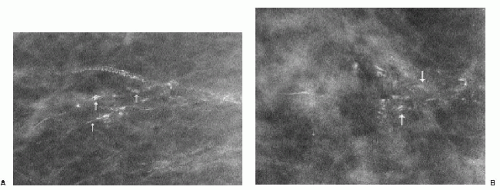
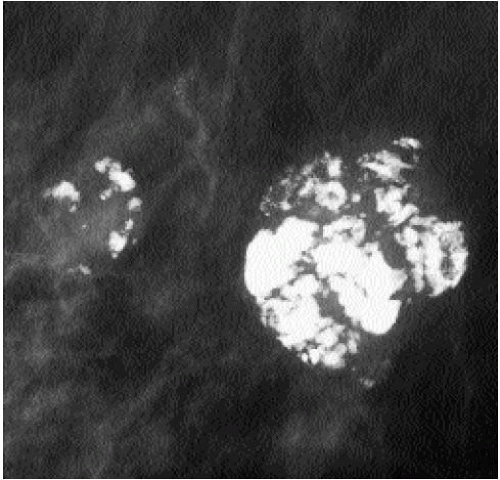
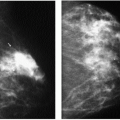
Popcorn-like calcifications (Figure 5.20) are dystrophic, developing in the hyalinized fibrous stroma of fibroadenomas. They are dense and coarse and can be unifocal or multifocal, unilateral or bilateral. In the initial stages of hyalinization with calcification, pleomorphic clusters may be seen with or without an associated mass (Figure 5.21). Sequential mammograms demonstrate progressive deposition of calcium with coalescence and formation of larger calcifications (Figure 5.22). In some women, these calcified fibroadenomas are palpable. It is imperative that the benign nature of the palpable finding be described and that patient and referring physician be assured, so that the likelihood of biopsy is minimized.
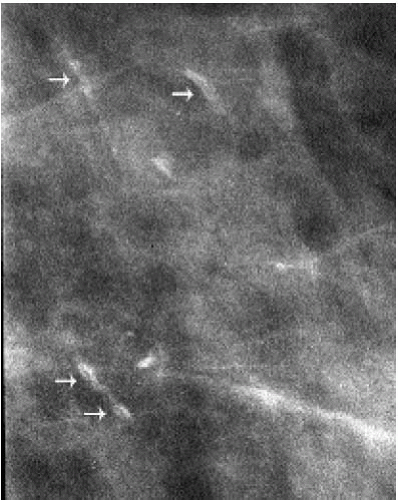 Figure 5.19 Dystrophic calcifications. Double spot compression magnification view demonstrating a cluster of linear calcification with central lucency. Categorized as benign. Stable on follow-up. |
 Figure 5.21 Dystrophic calcifications. Sequential craniocaudal views demonstrate the development of two clusters of calcifications. Other, similar clusters are present in the contralateral breast (not shown). A. Initial mammogram, showing foci of early calcification (arrows). B. One year later, additional calcifications have developed in each of the two clusters shown. C. Two years after the initial mammogram, additional calcifications have developed. D. Three years after the initial mammogram, some of the calcifications are coalescing and forming coarse calcifications. As fibroadenomas hyalinize, calcifications can develop in the stroma; as the calcifications coalesce, popcorn-like calcifications are formed. Notice the jagged edges or acute angles on some of the curvilinear calcifications (arrows). Category 2.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|