Diagnostic Breast Imaging
APPROACH TO DIAGNOSTIC BREAST IMAGING
The diagnostic patient population is composed of two groups of patients: those with abnormal screening mammograms requiring additional imaging evaluation, and those who have signs and symptoms of breast disease. Different approaches to diagnostic mammography are available. Our approach has been to develop a consultative breast service such that we run the center more like a clinical practice than a radiology service. We believe that as breast imagers, we are in a unique position to provide a desperately needed comprehensive breast health service. In many communities, breast care is disjointed and provided haphazardly with no standardization by general surgeons, gynecologists, family practitioners, and internists. Having someone who can put things together is quite helpful for patients. As radiologists, we have at our disposal all of the imaging modalities that are critical in the diagnosis and subsequent management of patients. If we complement this with clinical acumen, we can provide a much needed service. Functioning as a team member, we can serve as the pivotal patient care point. We have elected to develop our service with these concepts in mind. The radiologist is a clinical breast imager. Our approach to patients with possible breast cancer is to evaluate them as needed to reassure them of benign findings or low likelihood of malignancy, or to undertake the necessary procedures to establish a definitive diagnosis in a timely manner.
The diagnostic patient population includes two major groups of women: those with potentially abnormal screening studies, and those with signs or symptoms of breast cancer.
As discussed in Chapter 2, women with potentially abnormal mammograms are scheduled for diagnostic studies in 15-, 30-, and 60-minute slots, depending on the abnormality detected at screening. Our approach to these patients is to do whatever it takes, on their return trip, to arrive at a definitive, justifiable recommendation. In some patients, this may take 1 or 2 additional mammographic images, whereas in others, additional mammographic images, correlative physical examination, ultrasound, and an interventional procedure (e.g., cyst aspiration, ductogram, fine-needle aspiration, or biopsy) may be indicated. For those patients in whom a biopsy is indicated, we offer the patient the option of having it done immediately. Unless requested by the patient, we do not reschedule them for ultrasounds, aspirations, ductograms, or biopsies. About 99% of patients opt for having the biopsy done that same day. The referring physician is always contacted and informed of the patient’s decision. Most patients appreciate being offered the option of having the biopsy done that same day.
Box 3.1: Imaging Algorithms
Symptomatic patient (30 years of age or older) (“lump,” focal tenderness, dimpling, nipple retraction)
Metallic BB placed at site of concern
Craniocaudal views
Mediolateral oblique views
Spot tangential view at site of concern
Physical examination
Ultrasound (except if completely fatty tissue is present mammographically)
Symptomatic patient (under 30 years of age; pregnant or lactating regardless of age) (“lump,” focal tenderness, dimpling, nipple retraction)
Physical examination
Ultrasound
Full mammogram done if cancer is suspected
Although there are many more projections available to us during diagnostic mammography (e.g., caudocranial, lateromedial oblique, and superolateral to inferomedial oblique views), I have purposely limited the discussion to those used most often. Having a handle on the material discussed in this chapter will enable you to evaluate most patients presenting to your diagnostic center in a logical and efficient manner. Appropriate and justifiable recommendations become self-evident, and confidence in your work is enhanced greatly.
Our approach to patients presenting for diagnostic studies is to do whatever is needed to arrive at a definitive, justifiable impression and recommendation. In some women, this may require one or several interventional procedures.
SYMPTOMATIC PATIENTS: IMAGING ALGORITHMS
Our imaging algorithms are listed in Box 3.1. In women 30 years of age or older presenting with a lump, focal tenderness, skin dimpling, nipple retraction, or other focal symptom, a metallic BB is placed at the site of concern, and CC and mediolateral oblique (MLO) views are obtained bilaterally. A spot tangential view at the site of concern is obtained. Correlative physical examination and an ultrasound are done, unless the tissue is completely fatty and there is no possibility that the area of concern is excluded from the image. In women with spontaneous nipple discharge, a ductogram may be done. We do not consider diffuse, cyclical tenderness as an indication for a diagnostic study; these women are scheduled for screening mammography.
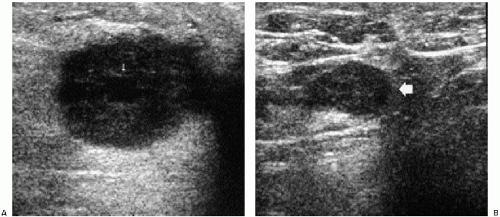
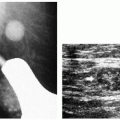
In symptomatic women younger than 30 years of age and those who are pregnant or lactating (regardless of age), a physical examination and ultrasound are undertaken (Figure 3.1). If cancer is suspected after this initial evaluation, a full mammogram is obtained.
SPOT COMPRESSION VIEWS
Spot compression views are the most common additional view obtained in our practice. The area of concern is maximally compressed and immobilized. This minimizes superimposition and geometric unsharpness, while resolution is improved as the object to film distance is decreased. When evaluating spot compression views, it is important to ensure that the area of concern is included in the field of view. As focal compression is applied, lesions can “roll” or “squeeze” out of the field of view. For purposes of orienting ourselves on the spot compression views, we like to include surrounding tissue on the image. Consequently, we do not cone-down on spot compression views, and, although not compressed, surrounding tissue is evaluated and used to ensure inclusion of the area of concern.
Spot compression views help minimize superimposition and geometric unsharpness and improve resolution by decreasing object-to-film distance.
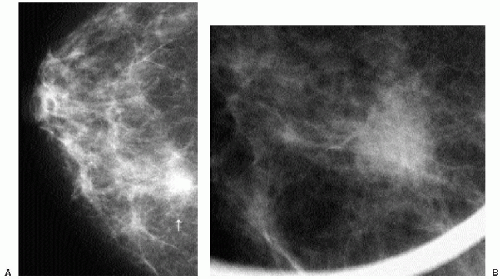
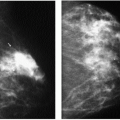
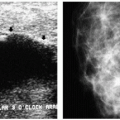
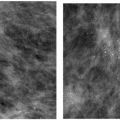
Indications for spot compression views are listed in Box 3.2. As a general rule, if we are not sure a lesion is present, our starting point is spot compression (Figure 3.2). Spot compression views are used to evaluate densities (seen in only one view) (Figure 3.3), masses whose margins may be obscured by surrounding tissue (Figure 3.4), asymmetric tissue (Figure 3.5), and possible areas of distortion. Additionally, spot compression (Figure 3.6) can be used to reach and image lesions that are excluded from routine screening views: lesions close to the chest wall, in the axillary tail, or high in the upper inner quadrants (1, 2, 3). If there is blurring related to inadequate compression (e.g., subareolar or inframammary fold areas) or focal areas of underexposed tissue, spot compression can help overcome these technical limitations.
Box 3.2: Indications for Spot Compression Views
Evaluation of questionable areas
Density
Asymmetry
Distortion
Evaluation of masses
When surrounding tissue may obscure margins
Inclusion of tissue
Posterior
Axillary
Upper inner quadrant
Technical issues
Blurring
Underexposure (focal)
Localizations
See Chapter 11
MAGNIFICATION VIEWS
Factors to consider associated with magnification views are listed in Box 3.3. Magnification views are obtained by moving the breast away from the image receptor (i.e., increasing the object-to-image distance and decreasing the source-to-object distance), thereby creating an air gap. A grid is not needed because scatter radiation is eliminated in the air gap. As the object-to-image distance increases, the amount of magnification increases (e.g., 1.5× and 1.8× common); however, this is associated with a loss of resolution from an increasing penumbra effect. The use of a small focal spot (0.1 mm) helps overcome the loss of resolution. With the small focal spot, however, exposure time is increased, leading potentially to motion. In an effort to obtain acceptable exposure times, the kilovoltage used to obtain magnification views can be increased by at least 2 from that used for routine views. Also helpful in shortening exposure times are magnification platforms made of Lexan (e.g., Mammo Spot). The magnification stands made of Lexan absorb about 20% less radiation than magnification stands with carbon fiber tops. Optimal exposures are facilitated as exposure times are decreased by 33% to 40%. A spot compression device is built into the surface of the Lexan magnification stands. If this is combined with a spot compression paddle, double spot compression can be obtained, maximally reducing breast thickness and further improving image quality.
Box 3.3: Magnification Technique
Air gap
No grid used (scatter radiation eliminated in air gap)
Small focal spot (0.1 mm) to overcome loss or resolution resulting from the penumbra effect
Small focal spot increases exposure times
Increase kV by factor of 2 from that used for screening images
Lexan top absorbs 20% less radiation than carbon top
Double spot compression to maximize compression
Spot compression paddle versus full paddle
For magnification views, consider increasing the kilovoltage used for routine views by at least 2. This helps minimize the effect of the lengthened exposure time that results from the use of a small focal spot (0.1 mm).
Magnification views can be done using the regular compression paddle or a spot compression paddle (1,4). With the regular compression paddle, a maximal amount of tissue is included on the image; however, compression may not be optimized. We use double spot compression for all of our magnification views (except those done for a ductogram). Using double spot compression at the site of mammographic concern maximizes compression and reduces exposure times, helping minimize the likelihood of motion. The disadvantages of using double spot compression relate to the smaller field of view and include the following:
Positioning needs to be accurate so that the area being evaluated is included in the spot view.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree