Well defined risk factors are smoke and alcohol consumption. Both cigarette and smokeless tobacco are strongly associated with the development of oral cavity, oropharyngeal, hypopharyngeal and laryngeal carcinomas [5, 6].
Betel and tobacco mastication, a practice much more common in eastern countries, is mainly associated with oral cavity tumours, while chronic wood dust inhalation seems to correlate with paranasal sinuses tumours [7].
The association of Epstein Barr virus (EBV) and nasopharyngeal carcinoma, especially with regard of undifferentiated histology is also known since long time.
More recently, an increase in diagnosis of oropharyngeal carcinomas has been documented, but only with regard to specific sites of the oropharynx. In detail, tonsillar and base of tongue carcinomas have became more frequent in the last decade, whereas other oropharyngeal carcinomas, such as those originating from tonsillar pillar and posterolateral wall, have maintained the same frequency over time [8]. This phenomenon has been associated with the increased incidence of human papilloma virus (HPV) positive tumours, which are much often oropharyngeal carcinomas and often arise from tonsil or base of tongue. HPV positive tumours are often diagnosed in male young adult (40–45 years old) with history of multiple sexual partners, non smokers or slightly smokers, and without history of alcoholism [9].
26.1.1 Classification: Onset Site and Histology
HNCs may arise from various sites of the cervico-facial region, including oral cavity, oropharynx, larynx, hypopharynx, paranasal sinuses and salivary glands. HNCs spread to laterocervical lymph node stations with a frequency variable from 10 % to 75 % [1]. Laterocervical lymphatic drainage reaches several stations which are classified according to Robbins (Fig. 26.1).
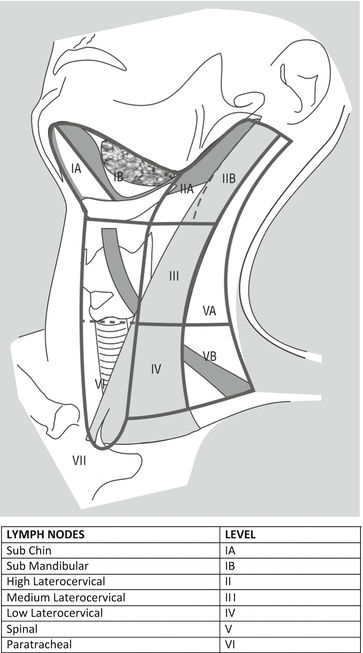

Fig. 26.1
Robbins classification of laterocervical lymph node levels (http://www.cancernetwork.com/cancer-management/head-and-neck-tumors)
Oral cavity can be divided into floor of mouth, upper and lower ridge, cheek mucosa, retromolar trigone, anterior tongue (comprising the anterior two-third), hard palate and lip. Oral cavity tumours are often squamous cell carcinomas and more rarely adenocarcinomas arising from minor salivary glands. Lymphatic drainage of oral cavity reaches laterocervical stations, in particular the I–III levels according to Robbins classification [10].
Oropharynx consists in different subsites, namely base of tongue, tonsils, soft palate and posterolateral pharyngeal wall. Squamous cell histology is strongly prevalent, representing more than 90 % of all oropharyngeal tumours; undifferentiated carcinomas are more rare. The oropharynx is extremely rich in lymphatics and a percent variable from 15 % to 75 % of patients with oropharynx carcinomas present laterocervical lymph node metastases at diagnosis. The main stations involved in these patients are the II–IV levels sec Robbins [11].
Hypopharynx may be divided in three areas, namely pyriform sinus, posterolateral wall and post-cricoids area. Almost all the hypopharynx tumours have a squamous histology. Lymphatic drainage of hypopharynx reaches the II–V levels sec. Robbins [12].
Larynx can be divided into supraglottic larynx, glottis and subglottis. Supraglottic larynx is further divided in epiglottidis, ari-epiglottic fold, and false vocal cords. Supraglottis structures are characterized by a rich lymphatic drainage, and often tumor arising from supraglottis present with early laterocervical metastases. The drainage lymphatic stations are the II–V levels sec. Robbins [13].
Glottic larynx is constituted by true vocal cords and anterior commessure. Glottis is not too rich of lymphatics and, with regard to T1 tumours, staging of neck is not recommended, given the low percent of lymph node metastases. Locally-advanced glottis tumours can spread to the II–V levels sec. Robbins [13].
Subglottis tumours are rare and often spread to III–VI levels laterocervical lymph nodes [14].
Nasopharynx is the anatomical region sited behind the nasal cavity. It is delimited on its upper side by the clivus and down by the pharyngo-basilar band. Tumours arising from nasopharynx are etiologically and prognostically different from other HNCs. About 40 % of them are undifferentiated carcinomas. The remaining 60 % are squamous cell tumours which can present a variable grade of differentiation, starting from well differentiated tumours, also known as keratinized carcinomas, until poorly differentiated tumours. Nasopharyngeal carcinomas are often diagnosed due to appearance of laterocervical palpable metastatic lymph nodes; the most frequently involved lymphonodal levels are the retropharyngeal, II-VI sec. Robbins [15, 16].
Tumors arising from the paranasal sinuses can occur in the frontal, mascellar and ethmoid sinuses, but nasal cavity tumors are also included in this category of HNCs. The most frequent histology is the squamous one, but mucoepidermoid, adenoido-cystic, undifferentiated and neuroendocrine tumours are also diagnosed in this anatomical region. Lymphonodal metastases are rare and are often related to undifferentiated sinonasal carcinomas (SNUCs) that represent the subtype with the poorest prognosis [17, 18].
Salivary glands tumours arise from both major and minor salivary glands. Major salivary glands carcinomas are much more frequent than those arising from the minor salivary glands, and are diagnosed in the parotid, submandibular and sublingual glands. Tumours arising from the minor salivary glands are mainly located in the hard palate. A wide number of tumors with different histology can be diagnosed in these organs. Among them, adenoido-cystic, mucoepidermoid, acinic, adenocarcinoma, squamous cell, malignant myoepithelial carcinoma are the most frequent. Lymphonodal metastases are rare except for specific histotypes, such as squamous cell carcinoma. Levels I–V lymph nodes can be involved [19, 20].
26.1.2 Settings of Presentation
Independently from the histology and the site of primary tumours, HNCs can be divided into three main disease presentation settings, including early, locally-advanced and recurrent/metastatic stage. Early stage disease comprises HNCs staged T1–T2 according to AJCC (American Joint Committee against Cancer). HNCs are rarely diagnosed at early stage, as they are characterized by few symptoms during their initial development. Locally-advanced HNCs are more frequent and are defined as T>2, and/or N-positive (N+) tumours, in the absence of systemic metastatic disease (M0). The third category is composed by newly diagnosed metastatic disease and recurring disease after primary treatment, which are both characterized by poor prognosis, and have similar treatment options [1].
26.1.3 Biology
For many years, alcohol and tobacco consumption have been the only known risk factors for HNC development. Recently, since the discovery of specific DNA mutations frequently detected in HNCs, it has been hypothesized that alcohol and tobacco may act as mutagens altering DNA in specific loci during the cancerogenesis process [21]. Indeed, tumours strongly related to alcohol and/or tobacco often show typical molecular features, such as TP53 mutation, Cyclin D1 upregulation, P16 downregulation, PI3KCA mutation and EGFR overexpression [22–24]. Patients affected are often male in their five to six decade of life, heavily smokers and/or drinkers. Moreover, these categories of HNCs are characterized by several chromosomal abnormalities and polyclonality, probably leading to poor sensitivity to both chemotherapy and radiotherapy.
On the other hand, HPV related tumours present the opposite features, showing often TP53 wild type status, overexpression of P16, down regulation of Cyclin D1 and low expression of EGFR, and are associated with a high proliferating index (Ki-67). Patients affected are young male or female (40–50 years old), non smokers or slightly smokers, and without history of alcohol consumption. HPV-related tumours are often oropharyngeal carcinomas, arising from tonsil or base of tongue and show a good response to both chemo and radiotherapy [25–27].
Basing on this evidence, it has been hypothesized that the cancerogenesis process may follow different routes, including an HPV-driven and an alcohol and/or tobacco-driven carcinogenesis. These two different types of carcinogenesis lead to tumors with completely different features, in terms of both prognosis and response to therapy.
Lately, many efforts have been put in treating HPV-positive tumours without aggressive therapeutic strategies. As matter of fact, several studies employing less toxic chemo-radiotherapy regimens, are ongoing and preliminary data are encouraging. Indeed, HPV-related neoplasms have shown to be much more chemo- and radiosensitive if compared with their HPV-negative counterpart [28–30]. In contrast, smoke and alcohol-related HNCs often show a variable grade of chemo- and radioresistance, as they are often characterized by peculiar DNA mutations leading to inhibition of apoptosis and stimulation of cell growth. PI3K-Akt pathway, for example, can be overactive in all HNCs, but this feature is much more common in HPV-negative, smoke and alcohol-related carcinomas. Importantly, the deregulation of the aforementioned pathway correlates with poor prognosis and poor response to radio- and chemotherapy [31, 32].
Recently, the pathway activated by programmed death-1 (PD-1) receptor and its ligand programmed death 1 ligand (PDL-1) has found to be hyperactive in oropharyngeal carcinomas [33]. PD-1 pathway regulates immune response during an inflammatory process. PDL-1 is exposed on the membrane of normal cells covering pharynx mucosa to avoid recognition and consequent destruction exerted by cytotoxic cells. Cytotoxic cells expose PD-1 protein which is able to link PDL-1 and avoid normal cell lysis. Importantly, some tumours utilize PDL-1 as a mechanism of escape from immunitary cell-mediated response.
RAS oncogene is very rarely mutated in HNCs having a frequency of 2 % or less, whereas NOTCH1 mutations have been reported to occur more frequently (10–15 % of HNCs) [34].
Mutations in both h-RAS and NOTCH encoding gene have been described, especially in patients with history of tobacco chewing and reiterated oral trauma. h-RAS and NOTCH-mutated HNCs show poor prognosis and poor response to conservative therapies (chemo and radiotherapy), though these data need further confirmation [34].
Most oral cavity and soft palate tumours often show poor prognosis and high rate of locoregional failure even after radical surgery. This feature has been linked to the locoregional immunosuppression status [35]. In fact, scientific evidence suggests a deficit in tumour infiltrating lymphocytes, due to the production of immunosuppressive cytokines by tumour cells, or in alternative, tumours cells may induce macrophages infiltrating tumour to produce these cytokines [36]. Therefore, restoring immune status may be taken into account for treating this category of tumours.
The totality of undifferentiated nasopharyngeal carcinoma and about 80 % of squamous carcinomas are EBV-related malignancies. EBV is able to provoke a latent infection in the infected cells and induce over time neoplastic transformation [37]. EBV-driven carcinogenesis is primarily due to the strong tumorigenic effect of some virus-related proteins, such as LMP-1 (latent membrane protein-1). LMP-1 is a transmembrane protein able to induce several downstream signals leading to cell proliferation, via NF-kB and cyclin-D pathway, immortalization, via telomerase activation, and angiogenesis [38–41]. EBV-related antigens, which are often expressed on cancer cell membrane, may be used as target for several strategies of immunotherapy.
26.1.4 Oral Cavity Tumours
Oral cavity has a rich lymphatic circle and regional node involvement is present at diagnosis in about 30 % of patients, being more common for some areas, including mobile tongue, and less frequently hard palate. Distant metastases are not very common at diagnosis, as a predominantly locoregional growth is the main feature of these tumours [1].
The main risk factors are oral trauma, smoking and smokeless tobacco. Diagnosis may be achieved after an accurate clinical exam of the oral cavity with a confirmatory biopsy and/or fine needle ago-biopsy (FNAB), which can be performed on both the primary site and on lymph node metastases. Staging program comprises a CT scan of the thorax and abdomen, which can be replaced by chest X-rays and liver ultrasonography, given the low incidence of distant metastases, especially in early-stage disease [1].
Early–stage disease is classified as T1-2 N0 M0 and surgery is its preferred treatment option. Sentinel lymph node biopsy was lately added to treatment strategy, since it can allow to spare elective neck dissection, reducing the morbidity associated with surgery. Nevertheless, sentinel lymph node biopsy should be employed only in centers with high level of expertise in this technique [42].
Radiation therapy is the alternative to surgery; it can be employed if the patients are considered unfit for surgery or if they refuse to undergo surgery. External beam radiotherapy is the most employed technique and it allows reaching doses up to 70 Gy on the clinical target. Lymph nodes are often included in the treatment plan and they receive a total dose of 50.4 Gy. Laterocervical levels I to III are often included in the treatment plan. The aforementioned doses are relative to a standard fractionating regimen with a daily dose of 2 Gy [43]. Hyperfractionated radiotherapy may allow to reach a higher total dose (82 Gy on the primary tumour and 63 Gy on the lymph node stations) [44]. Intensity Modulated Radiation Therapy (IMRT) should be considered the standard of care, when feasible. Interstitial brachytherapy has an important role in early-stage oral cavity tumours, especially if they have limited size (<2 cm) and are not involving bony structures, such as alveolar ridge [45].
Locally–advanced tumours are often treated with an integrated strategy comprising surgery, chemo- and radiotherapy. Of note, oral cavity tumours benefit from surgery even in the presence of a wide primary extension and massive nodal involvement. In this case, surgery is always followed by adjuvant treatment with either radiation alone or chemoradiation [46]. Adjuvant treatment is indicated in the presence of one or more risk factors defined after upfront surgery. Risk factors can be divided into major factors, including surgical margin involvement and extracapsular nodal spread, and minor factors, such as N2, T3 and invasion of perineural spaces. The presence of at least one major risk factor represent an indication for adjuvant concurrent chemo-radiotherapy, whereas in presence of one or more minor risk factors, radiotherapy alone is the preferred treatment [47–49].
In presence of unresectable or inoperable disease, concurrent exclusive chemo-radiotherapy can substitute upfront surgery. Wide carotid invasion, masticatory space involvement and prevertebral infiltration represent inoperability criteria.
Recurrent/metastatic disease is often treated systemically with chemotherapy, with or without palliative loco-regional treatments such as, radiotherapy, electro-chemotherapy and surgery [50]. Table 26.2 summarizes the treatment option by stage, for oral cavity tumours.
Table 26.2
Oral cavity tumours: treatment option by stage
Stage | Treatment options |
|---|---|
Early stage (T1–2 N0 M0) | Surgery (preferred) |
Exclusive RT | |
Interstitial brachytherapy | |
Locally advanced (T1–4 N0/+ M0) | Surgery followed by adjuvant RT +/− concurrent chemotherapy (Cddp-RT) (preferred) |
Concurrent chemoradiotherapy (Cddp-RT) | |
Concurrent Cetuximab-RT (poor PS patients) | |
Recurrent/metastatic disease | Re-surgery (if feasible) +/− chemotherapy (Cddp-5FU-Cetuximab) |
Chemotherapy (Cddp-5FU-Cetuximab) (preferred) associated or not with: | |
Palliative surgery | |
Palliative RT | |
Electrochemotherapy |
26.1.5 Oropharynx Tumours
Oropharynx is particularly rich in lymphatics, thus laterocervical metastases appear in 20–75 % of patients at diagnosis, especially in locally-advanced disease [51]. Diagnosis often requires fiberoscopy followed by biopsy or FNAB. Laterocervical lymph nodes are recurrently chosen for biopsy or FNAB. Staging is performed with total body CT scan.
About 60/70 % of oropharyngeal carcinoma are HPV-positive. However, currently it is not clearly known if determination of HPV status, using both in situ hybridization and immunohistochemistry for p16, may have an impact on the therapeutic management of the patients [1]. HPV positivity seems to be a good prognostic factor and some lines of evidence suggest its potential role as marker predictive of good response to primary chemotherapy [52, 53]. Moreover, clinical trials have shown a good outcome of HPV-positive patients even when treated with a less intense therapy [28–30], though this strategy is not currently standard according to both American and European guidelines.
Early–stage tumours (T1-2 N0M0) can be treated with both surgery and exclusive radiotherapy. Surgery options are transoral excision and more rarely open pharyngo-tonsillectomy. Selective laterocervical dissection (Level II–IV sec Robbins) is strongly recommended, and it should be performed bilaterally in presence of central mass or ipsilaterally in presence of a well lateralized primitive tumour [54].
External beam radiation therapy has the same efficacy of surgery in early-stage tumours and the most employed technique is IMRT, reaching a total dose of 80 Gy on the primitive and a prophylactic dose of 70 Gy on bilateral laterocervical lymph nodes (Level I–IV). When possible, IMRT should be employed in site of conformal 3D radiation therapy.
Locally–advanced disease is usually managed with a conservative approach, since it is often considered a systemic disease with capability of spreading to both locoregional lymph nodes and distant sites. American and European guidelines consider concomitant chemo-radiotherapy as the best option, with IMRT preferred over a conformal 3D technique [55]. A total dose of 70 Gy with a fractionating dose of 2 Gy should be employed. In addition, a higher daily dose (2.25 Gy), including a concomitant boost given on total tumor volume, may be used [55]. Recent data suggest that induction chemotherapy may be more effective than chemo-radiotherapy in patients with HPV-related disease, especially in presence of a particular genetic signature characterized by P16 overexpression, as well as by normal expression of cyclin D1 and high Ki-67. These tumours might benefit from upfront chemotherapy, representing a highly chemosensitive disease. However, further studies are warranted to demonstrate this hypothesis. Surgery has a lower grade of recommendation, especially in T3/4 and/or N2/3 disease. Nevertheless a surgical approach may be employed to remove residual disease after a conservative strategy.
Recurrent metastatic disease is normally treated with cetuximab-based first-line regimens including also chemotherapy, with or without locoregional palliative approaches, such as surgery, re-irradiation and/or electro-chemotherapy. Ongoing clinical trials are evaluating the possibility to use targeted therapy strategy, employing an anti PD-1 molecule, considering that the PD-1/ PDL-1 pathway is particularly active in oropharyngeal carcinomas [33]. Table 26.3 summarizes the treatment option by stage, for oropharyngeal carcinomas.
Table 26.3
Oropharyngeal carcinomas: treatment options by stage
Stage | Treatment options |
|---|---|
Early stage (T1-2 N0 M0) | Surgery (preferred) |
Exclusive RT | |
Locally advanced: T1-3 N0/+ M0 | Concurrent chemoradiotherapy (Cddp-RT) (preferred) |
Induction chemotherapy followed by RT +/− chemotherapy (Cddp or CBDCA or Cetuximab) | |
Concurrent Cetuximab-RT (poor PS patients) | |
Surgery followed by concurrent chemoradiotherapy (preferred) | |
T4 anyN M0 | Concurrent chemoradiotherapy (Cddp-RT) |
Concurrent Cetuximab-RT (poor PS patients) | |
Induction chemotherapy followed by RT +/− chemotherapy (Cddp; CBDCA; Cetuximab) | |
Recurrent/metastatic disease | Re-surgery (if feasible) +/− Chemotherapy (Cddp-5FU-Cetuximab) |
Chemotherapy (Cddp-5FU-Cetuximab) (preferred) associated or not with: | |
Palliative surgery | |
Palliative RT | |
Electrochemotherapy |
26.1.6 Hypopharynx Tumours
Hypopharynx is the transition tract between oropharynx and cervical esophagus and it is divided into three parts, namely pyriform sinus, posterolateral wall and post-cricoid area. Lymphatic drainage reaches the II–V levels sec Robbins and laterocervical metastases are particularly frequent at diagnosis [56]. Approximately 60 % of newly diagnosed patients have locally-advanced disease. Clinical neck exam and fiberoscopy are mandatory. Endoscopy should be followed by primitive lesion biopsy. In alternative, pathologic diagnosis can be made with a FNAB of lymph node masses. For staging, CT scan is employed. Positron emission tomography (PET) and bone scan should be considered only as second level exams [56].
Early–stage disease can be effectively cured with either surgery or exclusive radiation therapy. The most widely employed surgical technique is the transoral excision. Nevertheless, some T2 disease requires total laryngo-pharyngectomy and permanent tracheostomy. Neck treatment consists in selective bilateral lymphoadenectomy (levels II–IV) [57].
Locally–advanced disease, as well as T2N0 tumours that require demolishing surgery, are treated with a conservative approach, based on chemo-radiotherapy. Standard option is the induction chemotherapy followed by radiation therapy or chemo-radiation. Clinical evidences are in favor of a taxane-based induction chemotherapy followed by concomitant cisplatin and radiotherapy. Locally-advanced hypopharyngeal cancer shows in clinical trials a fairly good response rate after both chemo- and radiotherapy. After conservative a strategy, residual T and/or N disease may persist, and surgical removal is the preferred option in this circumstance [58].
Recurrent/metastatic disease is commonly treated with exclusive cetuximab-containing chemotherapy regimen. Table 26.4 summarizes the treatment option by stage, for hypopharyngeal tumours.
Table 26.4
Hypopharynx tumours: treatment options by stage
Stage | Treatment options |
|---|---|
Early stage T1-T2 (not requiring total laryngectomy) N0 M0 | Surgery (preferred) |
Exclusive RT | |
Locally advanced T1/3 N0/+ M0, (also T2 N0 requiring total laryngectomy) | Induction chemotherapy followed by RT +/− chemotherapy (Cddp; CBDCA, Cetuximab) (preferred) |
Concurrent chemoradiotherapy (Cddp-RT) | |
Surgery followed by RT +/− chemotherapy (Cddp) | |
Concomitant Cetuximab-RT (poor PS patients) | |
Surgery followed by concurrent chemoradiotherapy (Cddp-RT) (preferred) | |
T4 any N M0 | Induction chemotherapy followed by RT +/− chemotherapy (Cddp; CBDCA, Cetuximab) |
Concurrent chemoradiotherapy (Cddp-RT) | |
Concurrent Cetuximab-RT (poor PS patients) | |
Recurrent/metastatic disease | Resurgery (if feasible) +/− chemotherapy (Cddp-5FU-Cetuximab) |
Chemotherapy (Cddp-5FU-Cetuximab) (preferred) associated or not with: | |
Palliative surgery | |
Palliative RT | |
Electrochemotherapy |
26.1.7 Larynx Tumours
Larynx carcinomas can arise from three possible subsites, namely supraglottis, glottis and subglottis, being the glottic tumours the most frequent. Bilateral lymph node metastases are common in supraglottic cancers, especially in the locally-advanced stage. On the other hand, glottic larynx is poor of lymphatics, and early-stage tumours rarely spread to laterocervical lymph nodes. The most commonly involved lymph node levels are the II–V sec Robbins [VI level (paratracheal station) frequently involved in subglottic tumours] [1, 14].
The diagnostic work-up includes fiberoscopy and biopsy of suspected lesions, followed by CT scan of the neck, chest and abdomen, only in case of T>1 staged tumours, being lymph node metastases rare in T1 glottic neoplasms [59].
Therapeutic options for T1–2 lesions (early stage) include radical surgery, which can be performed by endoscopic laser excision or supraglottic laryngectomy [60]. Radiation therapy can be effectively used alternatively to surgery, though at stage III (T>2 and/or N+) chemo-radiotherapy is preferred to radiation alone.
In locally–advanced supraglottic tumours, except for T4 disease, conservative approaches, such as concurrent chemo-radiotherapy and preservation organ protocols, consisting in induction chemotherapy followed by chemo-radiotherapy or radiation alone, should be preferred to surgery. On the other hand, T4 tumours should be treated with radical surgery, consisting in total laryngectomy associated with bilateral neck dissection and in some cases with thyroidectomy [61], followed by adjuvant chemo-radiotherapy.
Recurrent/metastatic disease should be treated with chemotherapy associated with several kind of palliation therapies, including palliative radiotherapy, bisphosphonates in the presence of bone metastases, electrochemotherapy, re-irradiation, and palliative surgery, when indicated [62]. Table 26.5 summarizes the treatment option by stage, for larynx tumours.
Table 26.5
Larynx carcinomas: treatment options by stage
Stage | Treatment options |
|---|---|
Early stage (T1-2 N0 M0) | Surgery (preferred) |
Exclusive RT | |
Locally advanced T1/3 N0/+ M0 | Induction chemotherapy followed by RT +/− chemotherapy (Cddp; CBDCA, Cetuximab) |
Concurrent chemoradiotherapy (Cddp-RT) (preferred) | |
Surgery followed by RT +/− chemotherapy (Cddp) | |
Concomitant Cetuximab-RT (poor PS patients) | |
Surgery followed by concurrent chemoradiotherapy (Cddp-RT) (preferred) | |
T4 any N M0 | Induction chemotherapy followed by RT +/− chemotherapy (Cddp; CBDCA, Cetuximab) |
Concurrent chemoradiotherapy (Cddp-RT) | |
Concurrent Cetuximab-RT (poor PS patients) | |
Recurrent/metastatic disease | Resurgery (if feasible) +/− chemotherapy (Cddp-5FU-Cetuximab) |
Chemotherapy (Cddp-5FU-Cetuximab) (preferred) associated or not with: | |
Palliative surgery | |
Palliative RT | |
Electrochemotherapy |
26.1.8 Nasopharynx Tumours
Nasopharyngeal carcinomas (NPCs) are rare in western countries and are often characterized by undifferentiated or poorly differentiated squamous cell histology. NPCs frequently show high propensity to spread to distant organs, in particular bone and lungs. Differently from other HNCs, surgery is not often employed, due to the tendency of this disease to spread systemically and to the major functional consequences associated with radical surgery performed on this anatomic site. Moreover, the rational for the use of chemo and radiotherapy-based approaches is justified by the high degree of chemo- and radiosensitivity of these tumours [1].
Early–stage NPCs, (T1) are effectively cured with radiotherapy alone. IMRT should be always employed and a total dose of at least 70 Gy should be reached on the target.
Locally–advanced disease (from T>2, including pharynx-basilar membrane involvement, to T4N3) should be treated with concurrent chemo-radiotherapy, or with induction taxane-containing chemotherapy followed by chemo-radiotherapy [63–65]. Addition of adjuvant cisplatin-5Fluorouracil chemotherapy, after upfront concurrent cisplatin-radiotherapy, has been largely employed in the past, but at present it represents only a 2A recommendation category, according to European and American guidelines.
Recurrent/metastatic disease often shows poor prognosis with a median survival of 6 months. The major therapeutic option is chemotherapy, which consists in cisplatin-5fluorouracil doublet for undifferentiated tumours, and cisplatin-5Fluorouracil-cetuximab for squamous cell carcinomas [66]. Immunotherapy may be employed only in the context of clinical trials, and its rationale is based on the frequent expression of several viral antigens, such as LMP-1, -2 and EBNA-1, -2, on the membrane of NPC cells. The most employed immunotherapy strategy is adoptive immunotherapy, achieved by generating a specific EBV antigen-restricted lymphocytes population, able to infiltrate tumour mass and cause tumour cell death. Cytotoxic specific EBV antigen-restricted T-lymphocytes are obtained isolating white blood cells from patient peripheral blood, and exposing them to antigen presenting cells (APCs) that have been pulsed with EBV antigens (LMP and EBNA). Interaction between APCs presenting EBV antigens and white blood peripheral cells, in presence of IL-2 leads to the generation of a specific T-Lymphocytes population LMP and EBNA-restricted, which selectively attack NPC infected cells, causing tumour shrinkage, after i.v. re-inoculation. Several techniques of immunotherapy are currently being tested in ongoing clinical trials [67–71]. Table 26.6 summarizes the treatment option by stage, for nasopharyngeal carcinomas.




Table 26.6
Nasopharynx carcinomas: treatment options
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





