Evaluation of cardiac computed tomography (CT) images requires a thorough understanding of normal cardiac anatomy and common anatomic variants. Technologic advances, including electrocardiographically gated multidetector CT scanners, submillimeter collimation, and gantry rotation times shorter than 0.35 seconds, allow image acquisition with high temporal resolution and isotropic voxels. This makes noninvasive, motion-free imaging throughout the cardiac cycle and a comprehensive examination of the heart possible. The use of postprocessing techniques, including multiplanar reformation (MPR), maximum intensity projection (MIP), volume rendering (VR), curved reformation, and cine imaging, enables detailed evaluation of cardiac structures. Given the broad application of cardiac CT to various clinical situations, the use of standard terminology to describe and localize cardiac structures and coronary segments is essential for accurate communication of examination results. This chapter reviews the normal cardiac anatomy on CT and common anatomic variants.
Cardiac Chambers: Right Heart
The right atrium (RA) is positioned anteriorly and to the right, to form the inferior right heart border. The inferior vena cava (IVC), superior vena cava (SVC), and coronary sinus (CS) empty deoxygenated blood into the RA. The RA consists of three parts: the vestibule, the appendage, and the venous part, also known as the sinus venosus.
The sinus venosus is the smooth portion of the posterolateral RA wall located between the openings of the SVC and IVC. The crista terminalis, a prominent fibromuscular ridge formed by the junction of the sinus venosus and primitive RA, separates the smooth muscle fibers of the sinus venosus posteriorly from the trabeculated muscle fibers of the atrial appendage anteriorly ( Fig. 12-1 ). The crista terminalis varies in size and thickness in different individuals and is significant in several forms of atrial tachyarrhythmias that may necessitate catheter-guided radiofrequency ablation. The crista terminalis gives rise to the pectinate muscles, which fan out anteriorly. The septum spurium, the largest anterior pectinate muscle arising from the crista terminalis, is prominent in most patients and should not be mistaken for an intraatrial mass. The crista terminalis corresponds externally with the terminal groove, also known as the sulcus terminalis, a fat-filled groove on the epicardial side of the atrium containing the sinoatrial (SA) node and the terminal segment of the SA nodal artery. The inferior border of the crista terminalis near the IVC orifice is indistinct. The superior aspect of the crista terminalis arches anterior to the SVC orifice and extends to the anterior interatrial groove, where it merges with the Bachmann bundle, a flat band of muscle fibers bridging the anterosuperior margin of the interatrial groove. The Bachmann bundle, the largest anatomic and preferential interatrial electrical connection, facilitates rapid interatrial conduction and maintains physiologic, synchronous atrial contraction.

The right atrial appendage (RAA) is typically pyramidal, with a wider base and slightly larger pectinate muscles compared with the left atrial appendage (LAA), which has a narrower, finger-like appearance ( Fig. 12-2 ). These features aid differentiation between the two appendages when situs is questioned. The RA vestibule is a smooth muscular rim that surrounds the tricuspid orifice.

The RA also contains electrophysiologically significant structures such as the SA node and atrioventricular (AV) node. The SA node is located within the subepicardium, at the superior cavoatrial junction, extending from the SVC along the crista terminalis toward the IVC. It penetrates the crista terminals musculature to lie in the subendocardium. The SA node surrounds the SA nodal artery, which is centrally located in 70% of cases. In catheter-guided radiofrequency ablation, CT is used to measure the crista terminalis thickness and demonstrate the approximate location of the SA nodal artery in the nodal tissue. The Koch triangle lies in the RA at the orifice of the CS and is significant because its midportion contains the compact AV node (fast pathway) and its base contains the slow pathway.
The eustachian valve is located at the junction of the RA and IVC and, in utero, directs inflowing blood from the IVC toward the foramen ovale. The eustachian valve is variably developed and may be large and muscular. This valve usually inserts medially onto the eustachian ridge, which forms the border between the CS and the oval fossa. The eustachian valve free border continues as the tendon of Todaro, which runs in the musculature of the eustachian ridge. The thebesian valve is located at the CS entrance into the RA and prevents blood reflux into the CS ( Fig. 12-3 ). The thebesian valve usually consists of a thin semilunar fold in the anteroinferior ostium rim.

The right ventricle (RV) is the most anteriorly located cardiac chamber and has a characteristic heavily trabeculated apex, papillary muscles, and septomarginal bands. A distinguishing element of the RV is the moderator band, also known as the trabecula septomarginalis or septomarginal trabeculation, which is a muscular band extending from the interventricular septum to the anterior papillary muscle base ( Fig. 12-4 ). The moderator band is part of the right bundle branch conduction system and contains the right AV bundle (also known as His bundle), along with one or more arteries supplied by the left coronary artery system. The moderator band artery most commonly originates from the second anterior septal artery from the left coronary system. Additional connections between the moderator band artery and the right marginal artery or right ventricular branches (which originate from the right coronary artery) create a potential protective anastomotic network between the right and left coronary systems.

The RV conus (also known as the right ventricular outflow tract [RVOT] or infundibulum), a smooth muscular infundibulum located directly inferior to the pulmonary valve (PV), provides the outflow tract for blood from the RV through the PV into the main pulmonary artery. In complex cases, the RV may be differentiated from the left ventricle (LV) by identification of the moderator band, the heavily trabeculated apex of the RV, a well-developed infundibulum, septal papillary muscles, and lack of fibrous continuity of the AV valve and outflow tract. RV short-axis diameter is measured on axial CT images at the level of the tricuspid valve (TV) as the maximum distance between the endocardial surfaces of the free wall and the septal wall. The maximum diastolic minor axis value for the RV on average measures 41.3 mm (± 6.8). An increased RV/LV diameter ratio in the setting of pulmonary embolism of greater than 1 can be seen as a result of right-sided heart strain, and a ratio greater than 1.5 may indicate severe strain and is associated with poorer prognosis.
Cardiac Chambers: Left Heart
The left atrium (LA) is located posteriorly to the left and, similar to the RA, consists of three parts: a vestibule, an appendage, and a venous portion. Although many common variants of pulmonary venous anatomy are recognized, as described later in the pulmonary vein subsection, the superior and inferior right and left pulmonary veins typically drain into the posteriorly located venous component of the LA. Most of the atrium is smooth walled, including the venous component, the vestibule, and the septum. The LA vestibular component surrounds the mitral valve (MV) orifice. The trabeculated LAA is derived from the primitive atrium and arises from the superolateral aspect of the LA, thus projecting anteriorly over the proximal left circumflex (LCx) artery. The LAA is more tubular and finger-like in shape, compared with the pyramidal RAA, and it has a narrower base. The LAA is a potential site for thrombus formation because of the narrow neck connecting it to the LA, although unmixed blood and contrast material in the LAA can mimic a thrombus or mass on CT. Compared with the RAA, the LAA pectinate muscles are smaller, ( Fig. 12-5 ). These muscles are continuous fibers oriented parallel to each other within the LAA and should not be mistaken for thrombus. Normal LA area (excluding the LAA and pulmonary veins) is less than 20 cm 2 . An area of 20 to 29 cm 2 is mildly enlarged, an area 30 to 40 cm 2 is moderately enlarged, and one greater than 40 cm 2 is severely enlarged. The superior wall or dome of the LA is thickest, measuring 3.5 to 6.5 mm, whereas the anterior LA wall, just behind the aorta, is thin and vulnerable to tear.

The LV is located posterior and left lateral to the RV and, although thick walled, lacks the heavy trabeculations of the RV. The LV demonstrates fine trabeculations and two papillary muscles, anterolateral and posteromedial, which are in continuity with the ventricular myocardium. The posteromedial papillary muscle arises from the LV lateral wall. The papillary muscles function as part of the MV apparatus to ensure proper functioning of the MV leaflets. The LV myocardium has an anterior and inferior wall best visualized on paraseptal (or vertical) long-axis views, and the septal, apical, and lateral walls are best visualized on the four-chamber (or horizontal long-axis) views. The short-axis views enable assessment of the basal (closest to the MV), middle, and apical portions of the LV myocardium. The LV short-axis diameter is measured on axial CT images at the level of the MV as the maximum distance between the endocardial surfaces of the free wall and septal wall. The maximum diastolic minor axis value for the LV on average measures 42.0 mm (± 6.5). The LV is normally thin at the very apex (1 to 2 mm), even in abnormally thick-walled hearts (apical thin point). The outflow of the LV is by the LV outflow tract (LVOT), through the aortic valve into the aorta.
Cardiac Valves
The excellent spatial resolution of multidetector row CT enables visualization of valve leaflet anatomy, chordae tendineae, and papillary muscles. Valve motion and function can be assessed semiquantitatively with CT, and for patients with single left-sided valve dysfunction, CT can be used to calculate regurgitant volume and orifice size. CT is advantageous with better spatial resolution than magnetic resonance imaging for detailed leaflet anatomy and for accurate and reproducible evaluation of valve calcification, which may be degenerative or seen in association with valve stenosis. Valve prostheses are usually well visualized with CT; however, depending on the type of valve replacement, streak artifacts resulting from metallic components may limit leaflet assessment.
Aortic Valve
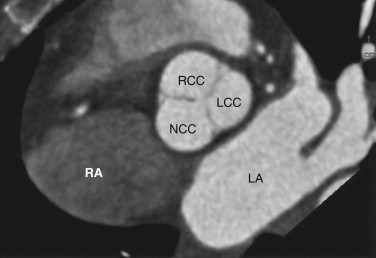
The aortic valve separates the LVOT from the ascending aorta and is composed of an annulus, cusps, and commissures (the area where two leaflets come together). This valve has no associated papillary muscles or chordae tendineae. The aortic valve is trileaflet and has right, left, and noncoronary (posterior) cusps ( Fig. 12-6 ). The right coronary cusp is located most anteriorly, the left coronary cusp most superiorly, and the noncoronary cusp most posteriorly, closest to the interatrial groove. The aortic cusps are half-moon shaped; thus, the aortic valve is also known as a semilunar valve. When closed, each cusp forms a pocket-like outpouching that opens into the ascending aorta and directs blood into the sinuses of Valsalva during diastole. The right and left coronary arteries arise from the right and left cusps, respectively. The aortic cusps are separated by three aortic commissures, roughly equally spaced around the valve annulus. The MV and aortic valve demonstrate fibrous continuity with each other, a feature that may assist in identifying the LV in patients with complex congenital heart disease.
Mitral Valve
The MV separates the LA and LV. It is the only bileaflet cardiac valve and is composed of anterior and posterior leaflets. The MV and its apparatus consist of an annulus, two leaflets, two commissures, two papillary muscles, and several chordae tendineae (linear fibrous bands) ( Fig. 12-7 ). Direct fibrous continuity occurs between the MV and the aortic valve through the MV annulus, a saddle-shaped fibrous ring embedded in the myocardium. This structure anchors the MV leaflets in continuity with the aortic annulus through three fibrous trigones, or intervalvular fibrosa. The MV annulus boundaries are normally not well visualized on cardiac CT; however, calcification is a common abnormality that permits its visualization. The free edges of the mitral leaflets are connected to the anterolateral and posteromedial LV papillary muscles through the chordae tendineae. During systole, the LV myocardium and papillary muscles contract, thus tugging on MV leaflets to ensure complete MV closure and to prevent prolapse.