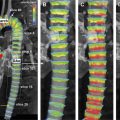
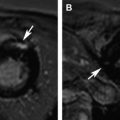
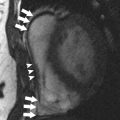
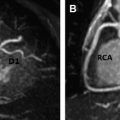
Patients with cancer are subject to short-term and long-term adverse cardiovascular outcomes from cancer therapies. It is important to identify patients at risk for cardiotoxicity so that appropriate therapy can be instituted early. Cardiovascular magnetic resonance (MR) imaging is emerging as a promising imaging modality with unique applications beyond standard left-ventricular systolic function assessment. It can provide comprehensive evaluation of most cardiac structures in one setting. This article provides an overview of cardiac MR imaging in cardio-oncology. Patients with cancer are at risk for developing short-term and long-term adverse cardiovascular outcomes, due to both direct and indirect off-target effects of cancer treatment. Cardiac magnetic resonance (CMR) imaging provides accurate assessment of left ventricular ejection fraction and should be used for monitoring cardiac function of patients both during and after cancer therapy. CMR imaging can additionally evaluate for myocardial, valvular, pericardial abnormalities; assess vascular compliance and provide tissue characterization of cardiac masses. Based on currently available estimates, there are 13.7 million patients with a history of cancer in the United States who are either cancer free or undergoing treatment, and about 1.7 million new cases are expected to be diagnosed in 2014. Owing to significant progress made in diagnostic and treatment capabilities over the past few decades, a sizable proportion of these individuals have an increased chance of long-term survival. Like diabetes or hypertension, cancer may now be considered as a chronic manageable disease and as such it requires not only early detection, periodic surveillance, and appropriate therapy but also control of comorbid conditions. Cardio-oncology has emerged as a unique interface between the two fields, because cardiovascular events are increasingly being recognized as major sources of morbidity and mortality in cancer survivors, because of either direct cardiac toxicity of chemotherapeutic agents or the presence of underlying concomitant cardiovascular comorbidities. Cancer can affect the cardiovascular system in multiple ways ranging from direct invasion of cardiac structures from primary or metastatic tumors of the heart to short-term or long-term cardiotoxic effects of treatment modalities, such as chemotherapy and radiation. The traditional focus of cardiac imaging has been on monitoring baseline and posttreatment left ventricular ejection fraction (LVEF), mostly through the use of echocardiography or multiple gated acquisition nuclear scans. Cardiac magnetic resonance (CMR) imaging, by virtue of its accurate depiction of cardiac morphology and function, is rapidly being adopted as an imaging modality that can provide a comprehensive assessment in cardio-oncology ( Fig. 1 ). This article discusses the role of CMR imaging in the following 4 broad categories: Cardiotoxicity of chemotherapeutic agents Radiation-induced heart disease (RIHD) Cardiac tumors Other conditions pertinent to cardio-oncology The occurrence of cardiotoxicity in the setting of chemotherapy can be variable and depends on the type of agent used, the overall cumulative dose administered, patient-related factors including age and comorbidities, as well as the use of adjunct therapies such as radiation. Of the different classes of chemotherapeutic drugs that are implicated in causing cardiotoxicity, anthracyclines and tyrosine kinase inhibitor toxicities are well described. In the case of anthracyclines, the incidence rates of acute, early-onset, and late-onset chronic progressive forms of cardiotoxicity are less than 1%, 1.6% to 2.1%, and 1.6% to 5% respectively. Incidence rates of cardiomyopathy and congestive heart failure greater than 36% have also been reported in patients receiving anthracycline doses of more than 600 mg per meter square of body surface area. In a clinical trial of women receiving trastuzumab, a monoclonal antibody–based tyrosine kinase inhibitor, for HER2-positive breast cancer, symptomatic congestive heart failure and asymptomatic 10% or more reduction in LVEF occurred in 1.7% and 7% of patients respectively. The incidence of trastuzumab-induced cardiotoxicity is even higher in real-life settings than is observed in clinical trials. The pathophysiologic mechanisms underlying chemotherapy-induced cardiotoxicity are complex and incompletely understood. Anthracyclines are thought to promote formation of free radicals, leading to myocardial injury, whereas inhibition of cardiomyocyte human epidermal growth factor receptor-2 resulting in ATP depletion and contractile dysfunction contributes to the cardiotoxic effects of trastuzumab. Despite deferring proposed pathophysiologic mechanisms, the end result ranges from clinically silent phenomena requiring coordinated surveillance through the use of biomarkers and cardiac imaging, to gross evidence of cardiac dysfunction with overt signs of heart failure. There is no universally accepted definition for chemotherapy-induced cardiotoxicity. A definition proposed by the Cardiac Review and Evaluation Committee supervising trastuzumab clinical trials relies on LVEF for identification of cardiac injury. By this definition, cardiac toxicity from cancer chemotherapeutic agents refers to a decline in LVEF by 5% or more to less than 55% in the setting of heart failure, or an asymptomatic reduction in ejection fraction by at least 10%. LVEF assessment by radionuclide imaging and echocardiography have been incorporated into clinical practice guidelines even though ejection fraction, by itself, may not be an accurate predictor of cardiotoxicity. In addition, significant measurement variability, as well as issues with methodology, including image quality, geometric assumptions, and dependence on loading conditions, may limit detection of small but important changes when using LVEF measured by echocardiography. CMR is considered to be the gold standard for the measurement of left ventricular volume, ejection fraction, and mass. Based on limited evidence from small, mostly single-center studies, it may have the capability to detect subtle changes in left ventricular volume and ejection fraction and increase in myocardial mass in the acute stage of early anthracycline-induced injury, presumably from cardiac edema. MR imaging also offers the unique advantage of characterizing tissues based on inherent differences in their magnetic properties (ie, T1, T2, and T2* relaxation times). In anthracycline-induced cardiotoxicity, there is potential expansion of extravascular space through myofibril loss, vacuolar degeneration, and inflammation. CMR offers an opportunity to detect myocardial inflammation and edema during early stages of cardiac injury. In a small, single-center study of 22 patients, early myocardial gadolinium enhancement on T1-weighted fast-spin echo imaging was linked to acute injury from anthracycline therapy and this was associated with significant reduction in LVEF during the first month of treatment. Early detection of myocardial edema by T2-weighted short-tau inversion recovery sequence, previously described as an indicator of acute injury in various disease processes, was also shown to predict a reduction in LVEF at 1 year in a preliminary single-center study involving 28 patients with breast cancer who received anthracyclines. Myocardial strain imaging is another promising CMR technique that can be used to assess early cardiac injury. In a proof-of-concept study involving 10 patients with hematologic malignancy treated with a regimen containing anthracyclines, 3-month posttreatment global circumferential strain, but not global longitudinal strain, was significantly decreased compared with pretreatment values. Note that data pertaining to the use of T1-weighted or T2-weighted imaging to evaluate early changes from cardiotoxicity, as well as strain imaging, are limited and require further studies to validate their clinical use for diagnostic or prognostic purposes. Given the traditional reliance on reduced LVEF for the diagnosis of cardiotoxicity and the ability of CMR to accurately quantify LVEF, it may be used to identify patients who could be missed when conventional imaging methods are used. A higher prevalence of cardiomyopathy was detected by CMR in adult survivors of childhood cancers treated with anthracycline, compared with two-dimensional echocardiography. CMR can also identify long-term cardiac sequelae of cancer chemotherapy such as left ventricular dysfunction, reduced left ventricular mass index, and right ventricular dysfunction. CMR is the imaging modality of choice to evaluate the presence of myocardial fibrosis, which is known to be a ubiquitous histopathologic feature of a broad variety of cardiomyopathies. Distinct patterns of late gadolinium enhancement (LGE) are recognized on CMR correlating with a specific cause. This is of particular importance during the assessment of chemotherapy induced cardiomyopathy, in individuals with preexisting cardiac conditions, such as, coronary artery disease ( Fig. 2 ). The prevalence of LGE in cardiotoxicity is low. Delayed enhancement patterns of the left ventricle seen on CMR include subepicardial LGE of the lateral, inferolateral, and septal walls in trastuzumab-induced toxicity; and midmyocardial LGE of the inferolateral wall and right ventricular insertion point in anthracycline-induced toxicity. Although LGE is the gold standard to identify focal areas of myocardial fibrosis, it is not well suited to assessing diffuse interstitial fibrosis, which is seen in cardiomyopathy caused by cardiotoxicity. Techniques such as native T1 mapping and quantification of extracellular volume (ECV) fraction may better depict diffuse fibrosis, and perhaps provide the ability to detect subclinical cardiac damage before decline in LVEF ( Fig. 3 ). Evidence is now emerging for the prognostic significance of increased ECV fraction in predicting hard cardiovascular events, including all-cause mortality in broad populations. However, robust data are lacking for its application in cardiotoxicity. In 30 pediatric patients (mean age, 15.2 years) with normal LVEF and 2 years or more after anthracycline therapy, increased ECV correlated with surrogate end points such as decreased left ventricular mass and wall thickness, lower peak oxygen uptake on cardiopulmonary stress test, and higher anthracycline cumulative dose. In another small single-center study of 42 adults (mean age, 55 years) who presented at a median time interval of 84 months following anthracycline therapy, diastolic dysfunction and increased left atrial volumes correlated with increased ECV. Radiotherapy is an essential component of contemporary cancer treatment and it is estimated that external beam radiation is used at least once in 52% of all patients with cancer. Inadvertent exposure of the heart to radiation during treatment of various malignancies involving the thorax and chest wall is considered to be the cause of RIHD. The aggregate incidence of RIHD is estimated at 10% to 30% by 5 to 10 years after treatment and injury seems to be potentiated by the use of concurrent chemotherapy. RIHD is a major nonmalignant source of mortality among long-term cancer survivors who have received radiation. Almost all cardiac structures are susceptible to radiation injury. Although not common, damage from ionizing radiation can manifest early as acute myocarditis and pericarditis. The late cardiovascular effects of radiation injury mostly include coronary artery disease (CAD) secondary to fibrointimal proliferation, classically affecting the ostia and proximal segments; myocardial fibrosis resulting in systolic and/or diastolic ventricular dysfunction; stenotic and regurgitant valvular heart lesions; conduction system disorders; and pericardial disease manifesting as chronic pericardial effusion or constrictive pericarditis. Given the wide spectrum of cardiac manifestations of RIHD, CMR offers a unique advantage to the clinician in being the comprehensive imaging modality. Cine imaging for cardiac anatomy and morphology, velocity-encoded or phase-contrast imaging for valvular function, T1 mapping/ECV quantification and LGE imaging for microscopic and macroscopic myocardial scar evaluation respectively, and stress perfusion imaging for the presence and extent of ischemia can provide valuable information in the clinical decision-making process. In long-term cancer survivors who have received radiotherapy, reversible perfusion defects are seen using single-photon emission tomography (SPECT). Because of the strong association between RIHD and increased major cardiovascular events, the high sensitivity and negative predictive value of stress MR imaging compared with SPECT in the evaluation of CAD may have some utility. A growing body of evidence supports the adverse prognostic implications of LGE in ischemic or nonischemic cardiomyopathies. Even though fixed perfusion defects are found on SPECT in individuals who have received mediastinal radiation, patterns of LGE and their prognostic implications need to be well defined in RIHD. LGE involving basal left ventricular segments can be seen in RIHD. Assessment of myocardial fibrosis by T1 mapping techniques is also potentially valuable in RIHD but their roles remain to be investigated. Another strength of CMR is in the evaluation of pericardial diseases, in which it offers integrated morphologic, functional, and physiologic data. This strength can be especially useful in differentiating between constrictive pericarditis and restrictive cardiomyopathy, which may overlap in RIHD. The combination of features favoring constriction include increased pericardial thickness, systemic venous dilatation, inspiratory septal bounce indicating ventricular interdependence, and tagged CMR depicting adhesions of pericardial layers. Despite the promise CMR possesses, hard trial evidence with respect to its use in the evaluation of RIHD is largely lacking. More studies are needed to accurately describe the prevalence, patterns, and prognostic significance of CMR findings in RIHD. Tumors of the heart, whether primary or secondary, are rare, with prevalence rates of 0.056% and 1.23%, respectively. Clinical manifestations of cardiac tumors are varied. They range from being incidental findings on imaging to causing constitutional symptoms and systemic embolic events, and because of their location they can present mimicking myocardial, valvular, or pericardial diseases. Primary cardiac tumors are largely benign. The most common are myxomas, followed by papillary fibroelastomas, fibromas, and lipomas. Primary malignant tumors of the heart are extremely rare and most of them are sarcomas, followed by lymphoma. Secondary or metastatic cardiac tumors have a 100 times higher incidence than primary neoplasms. The common sources are carcinomas of the lung, breast, esophagus; hematologic malignancies; and melanoma. In contrast with other imaging techniques, CMR offers unique advantages in the evaluation of cardiac masses. Tumors generally contain larger cells and show more reactive inflammation compared with normal tissue, and hence have higher free-water content and longer T1 and T2 relaxation times. This difference creates the inherent contrast that differentiates normal and neoplastic cells by MR imaging. CMR gives accurate anatomic definition and tissue characterization using T1-weighted, T2-weighted, and gadolinium-enhanced sequences, whereas the functional impact of the mass can be assessed by cine imaging. The malignant or benign nature of a cardiac mass can be assessed using CMR in many cases. Tumor size greater than 5 cm, infiltration of adjacent structures, and the presence of pericardial or pleural effusions are highly specific but less sensitive features of a malignant process, whereas location outside the left heart, tissue inhomogeneity, and contrast enhancement are highly sensitive but less specific features ( Fig. 4 ).
Key points
Introduction

Cardiac magnetic resonance in the detection of cardiotoxicity from chemotherapeutic agents
Brief Overview of Cardiotoxicity
Detection of Early Cardiac Injury
Detection of Late Cardiac Injury


Cardiac magnetic resonance in radiation-induced heart disease
Cardiac magnetic resonance in the assessment of cardiac masses