(1)
Department of Nuclear Medicine, Kuwait University, Safat, Kuwait
Abstract
The heart consists of muscle, valves, specialized tissue, coronary arteries, and pericardium. In the embryo, during the first month of gestation, a primitive straight cardiac tube is formed. The tube comprises the sinoatrium, the bulbus cordis, and the truncus arteriosus. In the second month of gestation, this tube doubles over on itself to form two parallel pumping systems, each with two chambers and a great artery. The two atria develop from the sinoatrium; the right and left ventricles develop from the bulbus cordis. Differential growth of myocardial cells causes the straight cardiac tube to bear to the right, and the ventricular portion of the tube doubles over on itself, bringing the ventricles side by side (Fig. 8.1) [1].
8.1 The Heart
8.1.1 Anatomical Considerations
The heart consists of muscle, valves, specialized tissue, coronary arteries, and pericardium. In the embryo, during the first month of gestation, a primitive straight cardiac tube is formed. The tube comprises the sinoatrium, the bulbus cordis, and the truncus arteriosus. In the second month of gestation, this tube doubles over on itself to form two parallel pumping systems, each with two chambers and a great artery. The two atria develop from the sinoatrium; the right and left ventricles develop from the bulbus cordis. Differential growth of myocardial cells causes the straight cardiac tube to bear to the right, and the ventricular portion of the tube doubles over on itself, bringing the ventricles side by side (Fig. 8.1) [1].
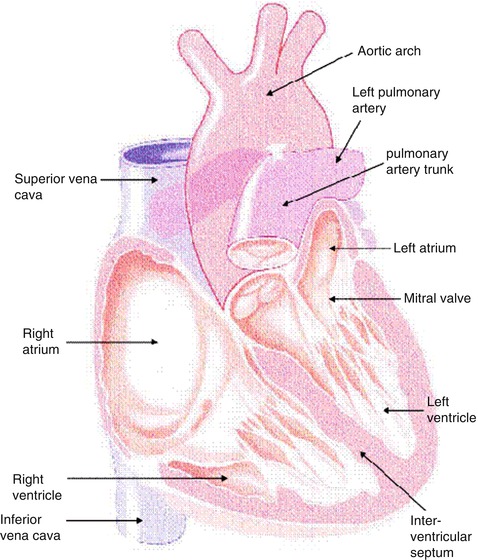

Fig. 8.1
Cutaway view of the heart
The coronary arteries originate from the left and right coronary sinuses of the aorta (Fig. 8.2). The left main coronary artery, which comes off the left coronary sinus, continues for a variable distance before it divides into two major arteries, the left anterior descending and circumflex arteries [2]. The left anterior descending artery (LAD) descends in the anterior interventricular groove and, most of the time, continues to the apex, supplying the apical and inferior apical portion. The LAD gives off septal branches that course deep into the interventricular septum. The septal branches vary in size and number. The anterior two thirds of the septum derives its supply from the septal LAD branches, while the rest of the septum is supplied by the perforator branches from the posterior descending branch of the right coronary artery. The LAD provides also diagonal branches, which run on the epicardial surface diagonally to supply the lateral wall of the left ventricle. Usually, the first one or two diagonal branches are large enough for angioplasty or bypass consideration.
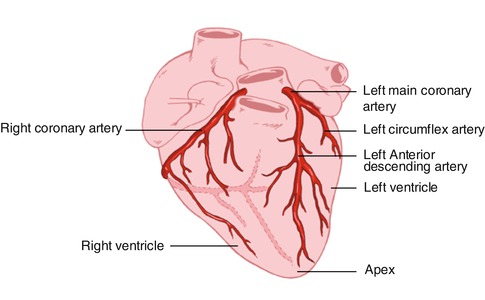

Fig. 8.2
Heart showing the origination of the coronary arteries from the left and the right coronary sinuses of the aorta
The left circumflex artery (LCx) branches off from the left main artery and runs in the left atrioventricular groove. It then continues to the left and posteriorly. It supplies several posterolateral ventricular branches, which in turn supply the posterolateral surface of the left ventricle and parallel the diagonal branches of the LAD. In most cases the LCx continues as a small terminal posterior left ventricular branch.
The right coronary artery (RCA) arises from the right coronary sinus and descends in the right atrioventricular (AV) groove. Its first supply is to the proximal pulmonary conus and right ventricular outflow region. Normally, there are also two or three large right ventricular branches that course diagonally over the right ventricle and supply the right ventricular myocardium. Most of the time the RCA continues along the diaphragmatic surface of the heart in the AV groove to reach the crux. At the crux the RCA divides into a posterior descending artery (PDA) and posterior left ventricular branch. The PDA branch is usually a large artery that runs in an anterior direction in the inferior interventricular groove. The PDA supplies the inferior third of the septum. The PDA septal branches can provide a rich collateral pathway via septal perforating arteries of the LAD. The other terminal branch of the RCA, the posterior left ventricular branch, continues in the AV groove and communicates with the terminal branch of the Cx.
8.1.2 Physiological Considerations
Cardiac muscle has two essential properties: electrical excitability and contractility.
8.1.2.1 Electrical Excitation of Myocardial Muscle
The conduction system is composed of modified cardiac cells. The sinoatrial and atrioventricular nodes have cells with high electrical impulse automaticity while the bundle of his and the Purkinje system cells have higher rapid impulse conductivity. The contraction of the heart is normally initiated by an impulse in the sinoatrial node, then spreads over the atrial muscles to the atrioventricular node. The impulse then runs through the His bundle and the Purkinje system to reach all areas of both ventricles at approximately the same time [1].
8.1.2.2 Myocardial Contraction
The ability of myocardial muscles to shorten and generate the force necessary to maintain blood circulation is a fascinating property of the heart. This is achieved primarily through the unique contractile function of two proteins of the sarcomere (actin and myosin) of the syncytially arranged myocardial fibers. The two main mechanisms that can alter cardiac muscle performance are a change in initial muscle length (Frank-Starling mechanism) and a change in contractile state. In the intact heart, these are determined by preload status, afterload status, the contractile state under a given set of loading conditions, and the heart rate. There is a passive exponential relationship between the length and the tension of muscle fibers. Cardiac muscle tissue, like other body tissue, is not entirely elastic. Thus, this relationship does not exist beyond certain muscle stretch limits. Additionally, there is an active proportional relationship between the initial length of myocardial muscle and the force generated by this muscle, again up to certain length limits [1].
Unlike skeletal muscles, cardiac muscle cells are connected to each other by intercalated disks and do not run the length of the whole muscle. Also, the heart muscle has a rich supply of the high-energy phosphate needed for the contraction. Therefore, it may not easily develop an oxygen deficit as skeletal muscle does when its work exceeds its oxygen supply. Cardiac sarcomeres are limited by the fact that they can be extended only to a certain limit (the optimum length of 2.2 μm) whereas sarcomeres of skeletal muscles can be stretched out beyond that. Finally, cardiac muscle has all-or-none twitch contraction and cannot be physiologically tetanized as skeletal muscle can.
8.1.3 Assessment of Left Ventricular Performance
8.1.3.1 Left Ventricular Function Curve
The left ventricular function curve usually refers to plotting of some of the LV performance measurements such as stroke volume or work against some of the preload indices such as pulmonary capillary wedge pressure [2]. This analysis requires invasive measurements and is useful not only for providing prognostic information in acute cardiac conditions but also for monitoring response to therapeutic interventions.
8.1.3.2 Ejection Fraction
The ejection fraction is the most useful single number of the LV performance, defined as the stroke volume divided by the end-diastolic volume. This functional index can be measured by both invasive and noninvasive techniques. Ejection fraction is closely related to the LV function curve; however, it is very sensitive to loading conditions [2].
8.1.3.3 Stroke Volume Index
By studying the pressure-volume relationship, a stroke work index can be obtained [2]. This is defined as stroke volume X (mean LV systolic ejection pressure–mean LV diastolic pressure). It is a very sensitive index since it is affected by all factors that may alter LV performance.
8.1.3.4 Regional Wall Motion Assessment
Assessment of regional wall motion is extremely useful in confirming and locating the site of coronary artery disease (CAD). As with LV ejection fraction measurement, it can be studied using both invasive and noninvasive methods.
8.1.3.5 Diastolic Function
Diastolic function is usually assessed by studying the relationship between LV passive pressure and volume and by examining the rate of relaxation after contraction. Several important measurements have been derived from various invasive and noninvasive techniques that can be used for both evaluating and monitoring the changes in diastolic function [2].
8.1.4 Pathophysiology of Cardiac Dysfunction
Heart failure is considered a pathophysiological condition rather than a specific disease. In such a condition, the heart fails to supply enough blood to meet the metabolic demand of the tissues. Most cases of heart failure are due to primary myocardial dysfunction or intrinsic abnormalities, which include hypertensive myocardial hypertrophy, ischemic heart disease, valvular heart disease, pulmonary hypertension, pericardial disease, and other cardiomyopathies (Table 8.1). Various extrinsic abnormalities can cause heart failure as well, despite normal ventricular function; this is referred to as secondary heart failure. Heart failure in this situation could have many reasons: inadequate blood volume as in hemorrhage, inadequate oxygen delivery as in anemia, inadequate venous return as in tricuspid stenosis, profound capillary vasodilation as in toxic shock, and peripheral vascular abnormalities as in arteriovenous shunts.
Table 8.1
Causes of heart failure
Systolic dysfunction |
1. Ischemic heart disease (e.g., chronic ischemia, myocardial infarction) |
2. Valvular heart disease (e.g., mitral regurgitation, aortic regurgitation) |
3. Dilated cardiomyopathy (idiopathic and nonidiopathic) |
4. Chronic uncontrolled arrhythmia |
Diastolic dysfunction |
1. Hypertension |
2. Ischemic heart disease (e.g., acute ischemia) |
3. Infiltrative myocardial disease (e.g., amyloid) |
4. Left ventricular outflow tract obstruction, e.g., hypertrophic obstructive cardiomyopathy, aortic stenosis |
5. Uncontrolled arrhythmia |
8.1.4.1 Hypertension
The main consequence of hypertension on the heart is an increase of the afterload pressure. Myocyte hypertrophy is the usual result, to add more new contractile proteins and mitochondria in order to maintain a normal cardiac output opposing the pressure overload [3]. On the level of molecular biology, stretching of myocytes by hemodynamic overload was observed to induce specific genes with a known growth regulatory effect such as proto-oncogenes [4]. These genes expand the myocyte capacity of protein synthesis, thus leading to concentric hypertrophy of the left ventricle (LV) without chamber enlargement. This process is often asymmetric in the various walls of the LV. In hypertrophic hearts, the increase in intracapillary distance and higher intracavitary pressure render the heart more susceptible to ischemia [5, 6].
8.1.4.1.1 Changes in LV Function with Hypertension
LV Diastolic Dysfunction
Diastolic dysfunction is usually evident long before the development of systolic dysfunction. A decrease in early peak filling rate and prolongation of the time to peak filling rate are seen in the majority of hypertensive patients [7]. Accordingly, a greater than usual atrial contraction contribution to the late diastolic filling is noticed as an effort to maintain a normal LV diastolic volume. A high LV filling pressure is thus seen in these patients and is then transmitted to the arterioles and capillaries of the lung. Therefore, hypertensive patients will start developing signs and symptoms of pulmonary congestion despite their normal ventricular ejection fraction [8].
LV Systolic Dysfunction
Long-standing LV pressure overload and the associated myocardial ischemia in the abnormally hypertrophic myocardium will eventually lead to a decrease in the heart’s ability to contract [9]. Congestive heart failure is the end result seen in almost all uncontrolled hypertensive patients.
Arrhythmia
In addition to total cardiac pump dysfunction, there is a significant increase in sudden cardiac death among patients with hypertrophic hearts. Both simple and complex ventricular arrhythmias develop more frequently than in nonhypertrophic myocardium, and this cannot be explained only by the usual coexistence of CAD in these patients [10].
8.1.4.2 Pulmonary Hypertension
The degree of pulmonary blood flow is affected mainly by the lumen size of pulmonary vessels [11]. Further, the pulmonary vascular resistance is defined as the difference of mean alveolar pressure and left atrial (LA) pressure divided by pulmonary blood flow. A change in any of these factors may therefore give rise to pulmonary hypertension. Pulmonary hypertension can be either primary or secondary to many other causes. In congenital heart diseases, increased medial thickening and atherosclerotic changes of the pulmonary vasculature are observed [12]. Such changes are also seen in patients with systemic to pulmonary collateral circulation. The sudden rise of PA pressure with irreversible RV failure and the usual significant decrease in LV systolic function association in acute pulmonary embolization are the cause of high mortality within the first hour in these patients [13]. Conversely, intimal fibrosis due to thrombus organization is the reason behind the cor pulmonale in chronic pulmonary embolization [14]. Pulmonary hypertension can also develop due to a rise of pulmonary venous pressure caused by LV diastolic dysfunction or high LA pressure. If such a condition persists long enough, medial thickening and arterialization of pulmonary veins will develop, which results in pulmonary fibrosis and destruction of alveolar capillaries [11]. The most common chronic lung disease associated with cor pulmonale is chronic bronchitis. The increased pulmonary vascular resistance in this case is caused by a reduction in the total area of the pulmonary vascular tree as well as mild thickening of the pulmonary arterioles [15].
Unlike the LV, the RV is a high-volume, low-pressure pump. Consequently, as pulmonary vascular resistance increases, a decrease in RV stroke volume and EF is observed [16]. An increase in heart rate does not usually provide enough compensation, and a decrease in cardiac output is inevitable. Additionally, signs and symptoms of systemic venous congestion are seen due to high-pressure transmission from the RV. Diastolic LV dysfunction due to RV failure could be caused by the decrease in both the LV distensibility and the myocardial blood flow from the accompanied elevation in coronary venous pressure [17].
8.1.4.3 Valvular Heart Disease
The valvular destruction in acute rheumatic fever is related to both humoral and cell-mediated immunological reactions, since the cell membranes of group A streptococcus antigens share common determinants with the heart [18]. Mitral valve regurgitation is the most common presentation of acute valvulitis while mitral stenosis is the usual chronic sequel of this disease. Varieties of autoimmune valvular lesions have also been described in many connective tissue disorders. Of the infectious causes, cardiovascular syphilis and infectious endocarditis are still recognizable. Other uncommon causes of valvular heart disease include congenital heart disease, ischemic heart disease, and cardiomyopathies.
8.1.4.4 Cardiomyopathies
8.1.4.4.1 Dilated Cardiomyopathy
Dilated cardiomyopathy is not a single disease but rather the final result of various types of myocardial insults. These insults can be viral or other infectious processes; exposure to cardiotoxins such as lithium, anthracyclines, and alcohol abuse; hypertension; pregnancy; and immune-mediated myocarditis. The dilated ventricles also show some degree of hypertrophy but not proportional to the degree of dilatation [19]. Occasional transmural scars, mural thrombi, and a variable degree of increased interstitial fibrous connective tissue can be seen. Mitral and tricuspid regurgitation are frequently noticed due to annular dilatation, lack of sphincteric contraction, and malalignment of the papillary muscle. Reduced systolic function with dilatation of one or both ventricles is the criterion for recognition. Symptoms appear when cardiac output cannot be compensated for or LV filling pressure becomes high.
8.1.4.4.2 Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is an idiopathic process that affects mainly the LV myocardium, but the right ventricle may also be involved. Other causes of myocardial hypertrophy such as systemic hypertension and aortic valve stenosis must first be excluded. The hypertrophy is asymmetric in most cases but it can be concentric. This process commonly involves the whole septum but it may be localized to the subaortic region. Extension into the anterolateral wall is occasionally seen. An apical hypertrophy variation is seen mainly in Japan. Rarely, only mid-ventricular hypertrophy is seen. Extensive myocardial fiber disarray with myocardial fibrosis involving mainly the septum is the typical histopathological feature. Patients with hypertrophic cardiomyopathy often have an ischemic myocardium due to the generalized arteriolar dysfunction. Sudden death from ventricular arrhythmias is common during the first decade of their life. Those who survive develop progressive LV diastolic dysfunction.
8.1.4.4.3 Restrictive Cardiomyopathy
Two types of restrictive cardiomyopathy are observed: a rare, noneosinophilic, or primary restrictive cardiomyopathy and a more common eosinophilic type. Of the eosinophilic restrictive cardiomyopathy, endomyocardial fibrosis is described in the tropical zones while Löffler’s endocarditis is seen in the temperate zones. The morphological features of the eosinophilic type include myocardial hypertrophy and significant endocardial thickening with plaques of collagen-rich fibrosis that vary in size. The eosinophilic myocardium will first show areas of necrosis that will progress to scarring with possible superimposed thrombi and finally end as thick myocardial fibrosis [19].
8.1.4.5 Pericardial Effusion
Pericardial effusion is considered to be present when the amount of fluid in the pericardial space exceeds 50 ml. Pericardial effusion can be associated with generalized processes not related to the pericardium, such as congestive heart failure, hypoalbuminemia, volume overload, and pulmonary hypertension. In most cases, however, it is related to a pericardial disease. The most common causes are post-myocardial infarction and uremic, neoplastic, and idiopathic pericarditis. The hemodynamic consequences of pericardial effusion depend on the rate at which the effusion is developing and the compliance of both the pericardium and the ventricles. With significant increase in the pericardial fluid pressure, the filling pressure of both ventricles may decrease, which subsequently leads to a decrease in cardiac output. This condition is called pericardial tamponade and in severe cases is associated with a high mortality.
8.1.5 Scintigraphic Evaluation of Cardiac Function
Radionuclide techniques provide both accurate and noninvasive means of evaluating cardiac function. Their role and clinical utility over the past 40 years are well established in the initial diagnosis of patients with suspected heart disease as well as in monitoring and deciding on prognosis in patients with known heart disease [20]. The accuracy of radionuclide ventriculography was recently found to be comparable to magnetic resonance imaging [21].
Although most ventricular function studies are performed with the patient at rest, exercise functional studies can be also done to assess regional and global myocardial contraction changes with stress. Two distinct types of studies can be performed either at rest or under stress: In the first-pass method, a bolus of radioactivity is dynamically imaged as it passes through the various vascular pathways of the heart; in the equilibrium methods, the heart is imaged over several hundred heart beats after an intravascular space radioactive tracer has reached equilibrium. The cardiac information obtained by these methods is summarized in Table 8.2 [22].
Table 8.2
Information obtained by radionuclide evaluation of ventricular function
1. Global right and left ventricular ejection fraction |
2. Regional right and left ventricular function |
3. Absolute ventricular volumes |
4. Systolic emptying and diastolic filling rates |
5. Detection and quantitation of cardiac shunts |
8.1.5.1 Imaging Methods
8.1.5.1.1 Equilibrium Radionuclide Angiography [ERNA]
Studies with radiopharmaceuticals require the use of an intravascular tracer that equilibrates within the blood pool. The ease with which 99mTc-pertechnetate can be attached to the patient’s own red blood cells (RBCs) makes labeled RBCs the preferred technique over labeled pooled human serum albumin. Labeling RBCs can be obtained by in vivo technique, modified in vitro technique, or in vitro technique. In vitro technique gives the best-quality imaging.
Assessing ejection fraction and regional wall motion requires measurement of volume changes and wall motion at different intervals throughout the cardiac cycle. Acquisition of multiple timed images of the blood pool activity in the heart will then be triggered by each R-wave. The duration of every frame may be 1–60 ms. Multiple beats are acquired to obtain adequate counts in each frame and typically a complete radionuclide ventriculographic study will consist of 200–800 summed beats for each of the three planar views [20]. There are three possible modes of acquiring ERNA: list, frame, and dynamic arrhythmia filtration. Each method has its advantages and disadvantages, as described below and summarized in Table 8.3. Frame mode studies are generally acquired for 16–32 frames. It is extremely important that patients be in a resting state during the heart rate sampling prior to starting acquisition and throughout acquisition. Major shifts in heart rate will cause many beats to be rejected and prolong the acquisition.
Table 8.3
Comparison between the different modes of computer acquisition
Mode of acquisition | Advantages | Disadvantages |
|---|---|---|
List mode | Optimal temporal resolution | Intensive memory requirement |
Excellent arrhythmia rejection | Longer processing time | |
Frame mode | Easy setup | Count drop-off |
Minimum memory | Fixed temporal resolution | |
Poor arrhythmia rejection | ||
Dynamic arrhythmia (buffered beat) mode | Flexible temporal resolution and arrhythmia rejection | Longer setup for greater options |
Less memory than list | ||
Accurate systole/diastole |
8.1.5.1.2 Exercise Radionuclide Angiography
Exercise radionuclide angiography is performed during supine or upright bicycle exercise. Bicycle exercise is necessary because treadmill exercise causes movement of the chest, and this does not allow acquisition of movement-free images for analysis. In the supine or upright bicycle position, the patient’s chest is placed against the camera in the best septal-separation LAO view, and the camera and patient are strapped together to further minimize movement during acquisition [23]. The patient is prepared for blood pressure and continuous ECG monitoring. Two or three ERNA are acquired in the resting state. Exercise is started at a workload of 25 W (150 kpm/min) and increased by 25 W at the end of each 3- or 4-min exercise stage. After the first minute of exercise, image acquisition is started and it continues for 2 or 3 min until the start of the next stage. As in treadmill ECG testing, exercise is terminated by symptom-limiting fatigue, angina, dyspnea, or observed life-threatening signs such as arrhythmia, hypotension, or marked ST segment depression. Slow bicycle pedaling against no load will help prevent post-exercise hypotension and leg cramps. At the end of exercise, two or three studies are obtained to document recovery of function [22].
Images are processed and ejection fraction is calculated using the same technique as rest ERNA. Serial ejection fraction is calculated for rest, each stage of exercise, and recovery. Visual assessment for both segmental wall motion and global function and size of the LV is an essential part of this examination.
8.1.5.1.3 First-Pass Radionuclide Angiography
Examination of the initial transit of a radionuclide bolus through the different major vascular compartments can provide information about the function of each chamber. This is probably the most accurate method of calculating RV ejection fraction and it is excellent for calculating LV ejection fraction as well. Optimal performance is achieved using a high count rate gamma camera interfaced to a high-speed computer. Multicrystal gamma camera systems offer the highest count rate capabilities but are not widely available [24]. The preferred radiopharmaceutical for rapid bolus injection is any 99mTc compound in a volume less than 1 ml and a dose of 15–30 mCi. Good-quality studies require that the radioactivity remain as a compact bolus to avoid overlapping of chambers’ radioactivity at any given time. If serial studies are essential, for example, at rest and following peak exercise, the initial resting study is done using an agent cleared rapidly by the kidneys (99mTc-DTPA or glucoheptonate) or liver (99mTc-sulfur colloid). The second study at peak exercise can then be performed using 99mTc-pertechnetate, which will allow in vivo labeling of the RBC pool and acquisition of ERNA views for assessment of regional wall motion during recovery.
Immediately following the bolus injection, 25–100 frames/s are acquired in the ANT or 30° RAO position. With multihead cameras, two or more projection images of the chest can be assessed simultaneously. The transit of the bolus can be evaluated either by ECG gating or continuous dynamic cine display. Using the gated first-pass technique, four to five individual beats can be summed to increase the number of counts in each frame; this provides better definition of valve planes and the lateral contours of the ventricle, which must be defined for ejection fraction calculation. Unlike equilibrium studies, cardiac chambers, especially the atria, can be studied individually with minimal interference from background or overlapping chambers. Because ERNA studies have difficulty in separating the RV from the RA due to overlap, the first-pass technique is considered the method of choice for accurate RV evaluation. Moreover, it is the only radionuclide method for detection and quantitation of left-to-right cardiac shunts [22].
8.1.5.1.4 Nuclear Probe and VEST
Nuclear probe and VEST are two systems that can be used in the acute care setting. The nuclear probe is a portable nonimaging device that allows calculation of beat-to-beat ejection fraction as well as diastolic function assessment that is comparable in accuracy to ERNA. It consists of a small sensitive radiation detector, a single-bore collimator, and a microprocessor. A major advantage of this probe is that it allows continuous cardiac function monitoring in the coronary care unit and for high-risk patients undergoing surgery or procedures that may affect cardiac function. Nonetheless, simultaneous visual examination of all cardiac chambers cannot be obtained [23].
The VEST is a similar nonimaging detector that can be attached to the patient’s chest over the heart and provides continuous beat-to-beat ejection fraction and ECG information that is recorded on a tape recording system similar to that used for Holter monitoring. Patients can ambulate and perform normal activities while their ejection fraction is being recorded. Newer VEST systems provide immediate, online ejection fraction measurements that allow identification of acute changes, and this makes possible the initiation of immediate treatment [22].
8.1.5.1.5 SPECT Gated Equilibrium Radionuclide Angiography
The advantages of this technique over the usual planar method include the ability to visualize each cardiac chamber without count contamination from adjacent structures. Regional wall motion can be also viewed in any projection. Additionally, the need for customizing the camera position to obtain the various planar views is overcome as patient has to be positioned once in the SPECT technique. Unlike planar ERNA studies, accurate computation of RVEF may be possible with SPECT ERNA due to the removal of chamber overlap and the 3D nature of SPECT. Finally, ERNA SPECT can be performed in approximately half the time used to acquire a 3-view planar ERNA series [25, 26, 29].
8.1.5.2 Clinical Applications
Nuclear medicine techniques are accurate and reproducible for cardiac function evaluation. They provide much important information that is useful in the diagnosis and management of the following clinical situations.
8.1.5.2.1 Assessment and Prognosis of Congestive Heart Failure
Congestive heart failure (CHF) is a leading cause of mortality and morbidity in the industrialized world. The primary causes are ischemic heart disease and hypertension. Other etiologies include valvular disease; viral, idiopathic, and alcoholic cardiomyopathy; and, less commonly, thyroid disease and hemochromatosis.
CHF stems from inadequate cardiac output, due to systolic or diastolic left ventricular (LV) dysfunction. This results in inadequate flow to the peripheral tissues at rest and with exertion, leading to compensatory responses designed to maintain perfusion pressure to vital organs.
In many patients, the severity of global ejection fraction impairment can be suggested on the basis of clinical and physical examination findings. In some, however, it is not always easy to separate right from left heart failure or the existence of both. Thus, the accuracy and reproducibility of serial measurements by ERNA are valuable and offer several advantages over other investigations.
In general, LV dysfunction with regional wall motion abnormalities, especially in the presence of normal RV function, is most consistent with an ischemic cardiomyopathy [27]. Such patients may benefit from antianginal therapy or evaluation for the presence of hibernating myocardium. Patients with severe biventricular enlargement and global dysfunction are less likely to have an ischemic etiology, and inflammation caused by viruses, exposure to cardiotoxins such as alcohol and adriamycin, or valvular disease should be considered.
ERNA is useful to differentiate between RV and LV heart failure. It is surprising how often clinical factors and physical examination findings do not allow a clear differentiation.
With the accurate functional information obtained from ERNA, particularly LV ejection fraction, more precise prognostic information about CHF patients is provided.
8.1.5.2.2 Cardiac Transplant Evaluation
ERNA has a role in the initial evaluation of patients being considered for cardiac transplantation. Initial ERNA evaluation of transplant candidates is essential to document the severity of ventricular dysfunction and the response to aggressive medical management, as well as to determine priority for transplantation based on the severity of impairment. Subsequent radionuclide ventriculograms are also helpful to monitor rejection and detect other postoperative problems.
8.1.5.2.3 Cardiotoxin Monitoring
Early in the clinical use of adriamycin, some patients receiving treatment developed a transient cardiomyopathy. Adriamycin also has a direct, dose-dependent cardiotoxicity that is progressive and sometimes fatal. This is seen particularly in patients with prior radiation therapy, concurrent cyclophosphamide treatment, and older age. Serial monitoring of ventricular function with resting ERNA was shown to be an accurate way of detecting early evidence of cardiotoxicity and allowed changes in therapy to prevent progression and in some cases to reverse the toxicity [27]. Endomyocardial biopsy is another accurate but expensive and invasive alternative for monitoring toxicity [28].
For adriamycin monitoring, a baseline study should be obtained prior to treatment to exclude clinically unsuspected heart disease. Patients with LV ejection fraction less than 30 % are at extremely high risk for toxicity and should probably not be started on adriamycin. Patients with ejection fraction between 30 and 50 % should have sequential studies before each dose. In these patients, an absolute decrease of ejection fraction greater than 10 % and/or a follow-up ejection fraction less than 30 % should be regarded as cardiotoxicity, and no further adriamycin should be given. With a baseline ejection fraction of greater than 50 %, a repeat study is recommended when the cumulative adriamycin dose exceeds 300 mg/m2 and before each subsequent dose when the cumulative dose exceeds 450 mg/m2. If there is a decrease in ejection fraction of less than 10 %, but the measured value remains more than 45 %, this is considered mild toxicity and therapy can be continued. Moderate toxicity is indicated by a more than 15 % decrease or a final ejection fraction less than 45 %, and adriamycin should be stopped. Repeat studies showing an improvement in ejection fraction may allow therapy to be restarted in these patients [22].
8.1.5.2.4 Assessment and Prognosis of Myocardial Infarction
ERNA has an important role in evaluating patients with myocardial infarction during the acute phase and for long-term follow-up.
The development of regional wall motion abnormalities is a manifestation of ischemia or infarction that precedes ECG changes and is a specific but not a very sensitive method. Thus, the presence of hypokinesis by ERNA in a patient with chest pain is consistent and specific for ischemia even in the absence of ECG changes [23].
In general, patients with myocardial infarctions show decreased global LV function that is directly related to the size of infarction. Additionally, in patients with CAD, the degree of resting LV cavity dilatation reflects mainly the extent of infarcted tissue and is closely related to the resting LV ejection fraction. It has been shown that ejection fraction measured during the first 24 h following infraction is the best predictor of in-hospital mortality. Patients with an ejection fraction less than 30 % have the highest mortality secondary to cardiac failure and arrhythmias. On the other hand, patients with normal or only mildly reduced ejection fraction have a much lower incidence of complications.
In patients who have suffered an acute myocardial infarction, the lower the ejection fraction at the time of discharge from the hospital, the shorter the long-term survival.
8.1.5.2.5 Preoperative Cardiac Risk Assessment
The widely used screening tests utilize the readily available clinical and physical examination findings of poor ventricular function. Congestive heart failure, rales, and an S3 gallop are the factors associated with the highest surgical risk by the Goldman and Detsky criteria. Because in some patients these findings are not adequately appreciated, an accurate, objective, and reproducible measure of function is desirable. It is well established that complications are increased during and following surgery in patients with a diminished LV ejection fraction.
The Coronary Artery Surgery Study also demonstrated that LV function was the best predictor of postoperative cardiac events in patients undergoing bypass surgery. In other studies, vascular surgery patients with an ejection fraction less than 35 % had increased cardiac complications and increased 30-day and 1.5-year mortality [29]. The increased surgical risk is due to the frequent coexistence of coronary artery disease. The incidence of cardiopulmonary complications in high-risk patients rises from 12 to 58 % in those with reduced radionuclide ventriculographic ejection fraction. Abnormal LV ejection fraction is an independent risk factor beyond the detection of myocardial ischemia with myocardial perfusion imaging [30].
8.1.5.2.6 Monitoring Valvular Heart Disease
Exercise ERNA is a very sensitive and noninvasive method to assess asymptomatic patients with aortic regurgitation and, to a lesser extent, mitral regurgitation for the preclinical detection of myocardial damage as manifested by a decrease in cardiac functional reserve [31]. A patient without myocardial damage will have a normal ejection fraction at rest and show increased ejection fraction by at least 5 % at peak exercise. Younger patients without clinical symptoms should have serial studies done every few years. Older patients or those with mild symptoms should be evaluated more frequently. Patients with aortic stenosis and an abnormal resting LV ejection fraction have a less favorable prognosis following aortic valve replacement.
Immediately following valve replacement, resting ERNA usually demonstrates improvement of the LV function. However, a decrease in ejection fraction can also be a normal finding due to the decrease in LV preload. Gradual improvement can be observed over time.
8.1.5.2.7 Myocardial Hypertrophy Evaluation
Myocardial hypertrophy due to hypertension, pressure/volume overloading, or idiopathic causes, e.g., hypertrophic cardiomyopathy, is associated with a decrease in early rapid ventricular filling. In patients with hypertension, such decreases may be observed before the development of obvious echocardiographic hypertrophy and may be an early sign of damage. Such changes can also be observed in aortic stenosis, but they are not clinically useful to monitor the need for valve replacement [22].
LV ejection fraction is usually in the higher normal value in these patients. This is presumably a compensation mechanism to maintain a normal stroke volume, as the hypertrophic LV is stiff and has smaller end-diastolic volume than the normal LV.
8.1.5.2.8 Cardiac Shunt Evaluation
Two distinctive types of studies can be obtained to both qualitatively and quantitatively evaluate cardiac shunts, depending on the type of shunt suspected.
Left-to-Right Shunt
A first-pass study should be performed to assess patients with this type of shunt. Subsequently, a time-activity curve is generated from a region of interest drawn in the lung field. The pulmonary transit time is normally shown as a narrow spike, with symmetric limbs that represent the pulmonary blood flow of the radioactivity. However, this curve, particularly the descending limb, is distorted in left-to-right shunts due to early recirculation of pulmonary blood from the shunt. Calculation of the pulmonary-to-systemic flow ratio can be obtained by subtracting the fitted shunt curve from the pulmonary one. This is a sensitive method for detecting pulmonary-to-systemic blood flow shunts between 1.2 and 3.0, provided that the patient has no pulmonary hypertension, congestive heart failure, or tricuspid regurgitation [22].
Right-to-Left Shunt
This can be suggested from visual examination of a first-pass study, where there will be early visualization of the LV. A more accurate and quantitative method of assessing these types of shunt is to inject 99mTc-macroaggregated albumin. The small particles of this radiopharmaceutical, used mainly in perfusion lung scan, are trapped in the capillary beds as they pass through the pulmonary arteries. However, in the presence of a right-to-left shunt, the pulmonary capillary system is bypassed and the particles enter the systemic circulation, where they are trapped in end organs such as the brain and the kidneys. Qualitative as well as quantitative analysis of activity within the body can be accurately obtained. A significant right-to-left shunt is present if the organ counts are greater than 7 % of the total lung uptake [23]. Complication because of capillary blockage is not a clinical concern with this procedure, as the number of particles used is very small compared with the number of capillaries in any organ.
8.1.6 Pathophysiology of Coronary Artery Disease
Coronary artery disease is almost always caused by atheromatous narrowing which may subsequently occlude the vessel. Early atheroma is present from young adulthood onward. A mature plaque is composed of two components, each associated with a particular cell population. The lipid core is mainly released from monocyte-derived mononuclear macrophages, which migrate into the intima and ingest lipids. The connective tissue matrix is derived from smooth muscle cells, which migrate from the media into the intima, where they proliferate and change their phenotype to form a fibrous capsule around the lipid core [32]. Currently atherosclerosis is viewed as an inflammatory disorder since inflammation has a major role in all stages of atherogenesis. When the arterial endothelium encounters certain bacterial products, its cells augment the expression of adhesion molecules that promote the sticking of blood leukocytes to the inner surface of the arterial wall. Once resident in the arterial intima, the blood leukocytes (mononuclear phagocytes) communicate with endothelial and smooth muscle cells, the endogenous cells of the arterial wall [33].
When a plaque produces more than 50 % diameter stenosis (or more than 75 % reduction in cross-sectional area), reduced blood flow through the coronary artery during exertion may lead to angina. On the other hand, acute coronary events arise when thrombus is formed following disruption of a plaque [32] as disrupted plaque represents a “solid-state” stimulus to thrombosis [33].
Atheroma is prone to disruption. The intimal injury causes denudation of the thrombogenic matrix or lipid pool and triggers thrombus formation. Additionally, sudden plaque growth occurs when the plaque ruptures, resulting in intracoronary thrombosis (Fig. 8.3). Occlusion is more complete in acute myocardial infarction than in unstable angina, where arterial occlusion is usually subtotal [32].
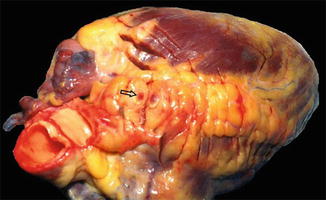

Fig. 8.3
Picture of a heart from a postmortem autopsy illustrating intracoronary thrombosis (arrow) (Courtsy of Prof. M. Elfawal)
Lowering of cholesterol plus the more recently appreciated anti-inflammatory effects of statins stabilizes the atheromatous plaque by decreasing the number of macrophages, increasing the net efflux of lipid from the plaque, and lowering indices of inflammation such as local temperature, uptake of FDG, and formation of a thicker fibrous cap. This helps make the plaque more resistant to disruption [34, 35]. Furthermore, the anti-inflammatory effects of statin therapy independent of cholesterol lowering appear to promote the stabilization of lesions in acute coronary syndromes [36].
Myocardial infarction occurs in the setting of an acute coronary vessel occlusion due to plaque rupture plus thrombosis [32, 33], with a variable amount of spasm and increased myocardial demand as contributing factors. Myocardial necrosis and severe acute ischemia lead to cellular membrane disruption and increased permeability. This injury result in leakage of a number of intracellular molecules used to detect tissue injury [37] and in large, exogenous-labeled molecules being able to penetrate and concentrate in these zones. Another area may consist of ischemia and/or hibernation, where myocardial contraction has ceased or diminished in response to decreased perfusion, but where cellular viability and membrane integrity are intact. Additionally, an area may have experienced severe ischemic injury, with partial or total spontaneous or therapeutic restoration of myocardial blood flow, but continued dysfunction due to ischemia-induced oxidative stress, or “stunned” myocardium. Finally, there may be a larger zone which is functional and perfused, but jeopardized by being supplied by a partially occluded artery. These various myocardial tissue states could be present in layers, or closely interdigitated with each other, seen pathologically as islands of viable tissue in the midst of fibrosis, or vice versa [38].
8.1.7 Scintigraphic Assessment of Coronary Artery Disease
Coronary artery disease management and follow-up can be aided by several radionuclide studies including myocardial perfusion SPECT imaging, myocardial perfusion PET imaging, exercise radionuclide angiography, radionuclide viability studies using conventional and positron emission radiotracers, and infarct imaging.
8.1.7.1 Myocardial Perfusion SPECT Imaging
Clinical manifestations of coronary artery disease include angina pectoris, myocardial infarction, congestive heart failure, and sudden death. It may be asymptomatic until advanced in severity or complications. Most diagnostic methods, both invasive and noninvasive, depend on detection of luminal narrowing of the coronary vessels. Increase of coronary flow caused by exercise or pharmacological stress exaggerates flow nonuniformity, through either increased metabolic demand or vasodilation. The easiest method of increasing coronary flow is physical exercise, using a motorized treadmill or a stationary bicycle. In patients who are unable to exercise adequately, pharmacological agents (adenosine, dipyridamole, dobutamine, and arbutamine) are used for transient elevation of coronary flow.
Myocardial perfusion imaging (MPI) maps the relative distribution of coronary flow, which is normally almost uniform in the absence of prior infarction or fibrosis. In the presence of luminal narrowing, flow nonuniformity corresponds to anatomical location of the coronary stenoses and to the cumulative severity of the obstructions along the coronary arterial tree and the size of its watershed [39]. Therefore, MPI can diagnose not only the presence of coronary artery disease but also its extent, severity, and physiological impact, thereby providing great prognostic information.
8.1.7.1.1 Stressors
Exercise
Exercise treadmill stress testing (ETT) is the most frequently used test for noninvasive diagnosis of coronary artery disease. Sensitivity of a symptom-limited ETT for diagnosis of CAD is 65–70 % [40]. When combined with myocardial perfusion imaging, sensitivity increases to 85–90 %, while specificity is increased as well [41]. Motorized treadmill exercise is the standard in the USA, while the upright stationary bicycle is more popular in Europe. All monitored parameters (i.e., ECG, blood pressure, patient’s appearance, and symptoms) are valuable not only for diagnosis but also for prognosis. Using a Bayesian approach, pre- and post-test probability for the presence of the disease can be reliably estimated. Short-term prognosis for major cardiovascular events can also be derived from easily obtained parameters. The most powerful single predictor in both men and women is exercise capacity (length of the exercise) [42, 43].
Pharmacological Stress Testing
Patients who cannot exercise for noncardiac reasons (e.g., orthopedic, neurological, peripheral vascular) or are unable to exercise adequately (for a meaningful period of time and/or to an adequate heart rate) are candidates for pharmacological stress testing. Four agents are currently approved for use in conjunction with MPI: adenosine, dipyridamole, dobutamine, and arbutamine. Adenosine and dipyridamole are coronary vasodilators. Dobutamine and arbutamine are beta-adrenergic agonists and increase myocardial oxygen demand. They also have some direct vasodilatory effect [44].
Adenosine
Adenosine is an endogenous coronary vasodilator produced from ADP and AMP in myocardial and vascular smooth muscle cells. Adenosine affects two kinds of receptors: A1 and A2. Activation of the A1 receptor slows AV conduction. Activation of the A2 receptor leads to coronary vasodilation (Table 8.4). The half-life of adenosine is extremely short (seconds only). Perfusion tracers are therefore injected during continuous adenosine infusion (140 μg/kg/min for 6 min). Side effects of adenosine include flushing in 37 % patients, chest pain in 35 %, shortness of breath in 35 %, and gastrointestinal symptoms in 15 %. Chest pain is not indicative of myocardial ischemia. Vasodilation causes a modest blood pressure drop, usually accompanied by compensatory tachycardia, although transient second degree AV block is seen in 3–4 % of patients and third degree AV block in <1 % of tested patients. All side effects and hemodynamic changes are transient and reversible. Use of an antidote (i.v. aminophylline) is rarely needed. Most situations can be controlled by decreasing the infusion rate and/or by shortening the duration of the infusion. Caffeine, theophylline, and their metabolites competitively block adenosine receptors. Therefore, patients should abstain from caffeine-containing beverages and medication for 12–24 h prior to the test [45, 46].
Table 8.4




Coronary vasodilators
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree