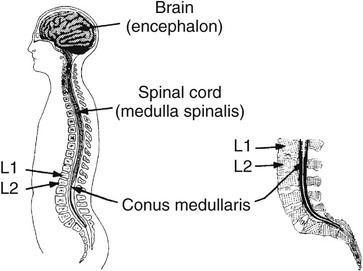
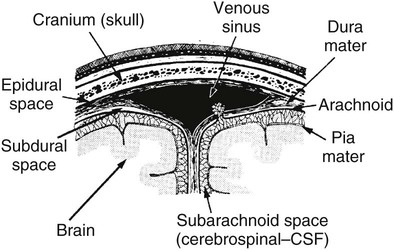
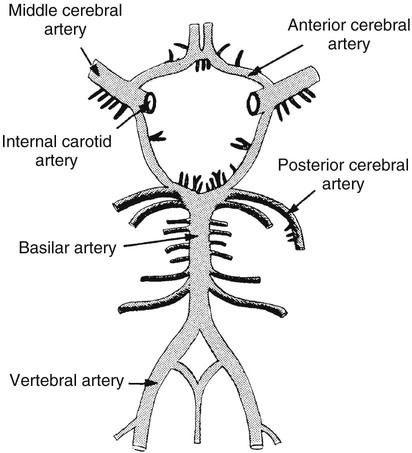
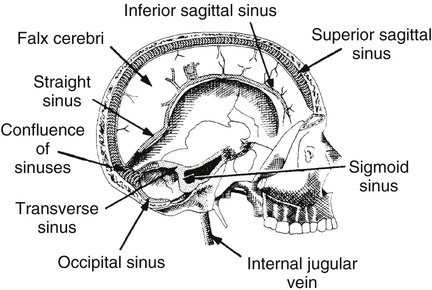
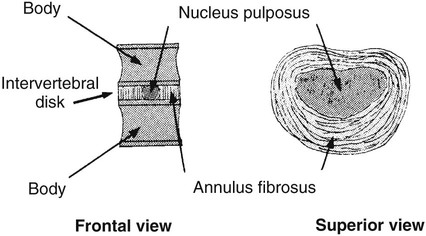
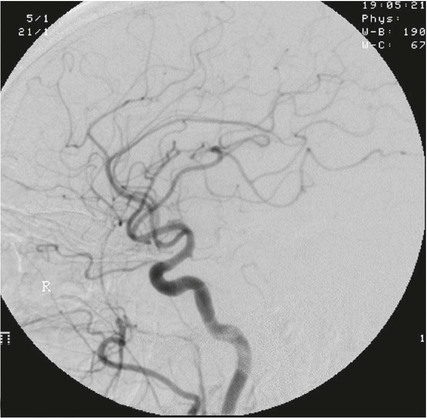
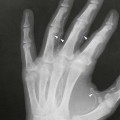
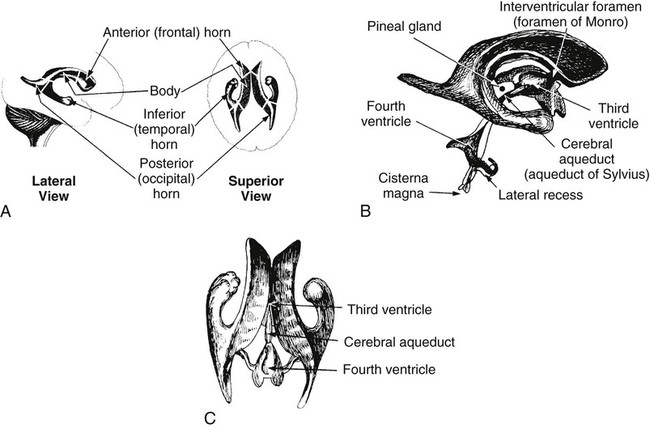
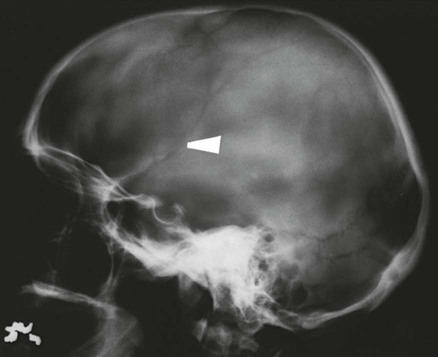
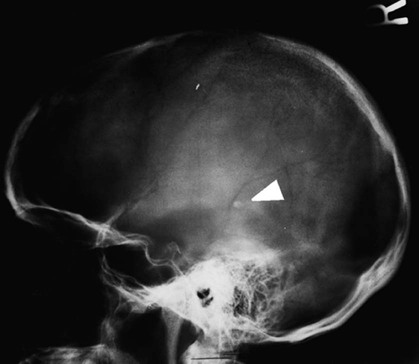
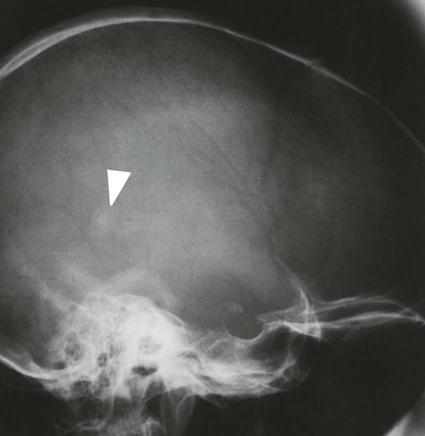
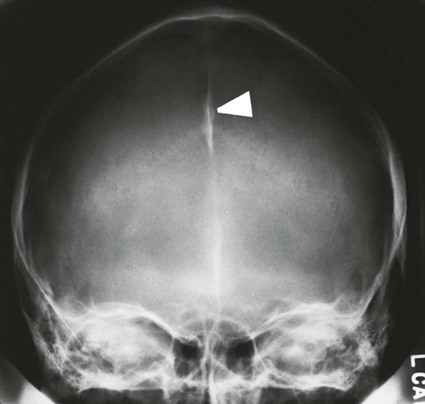
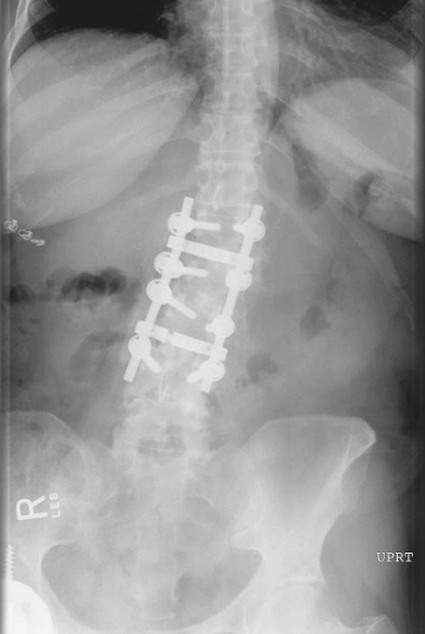
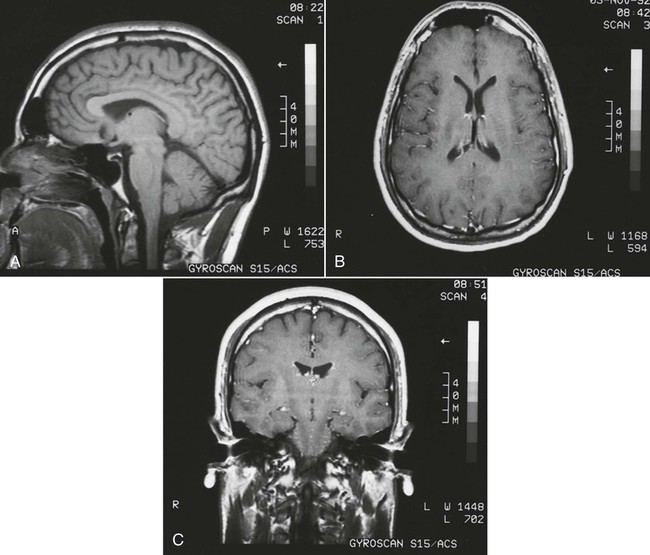
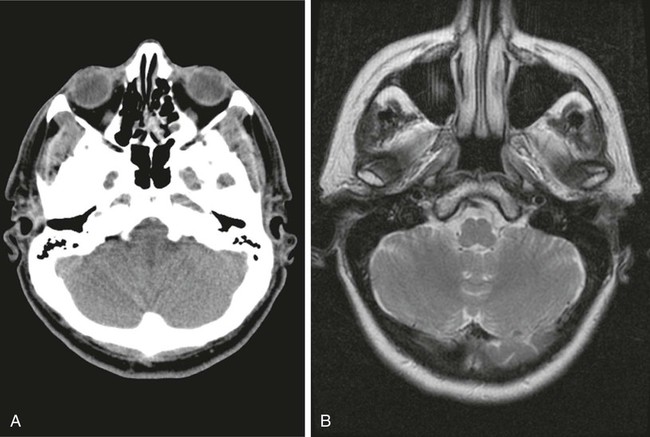
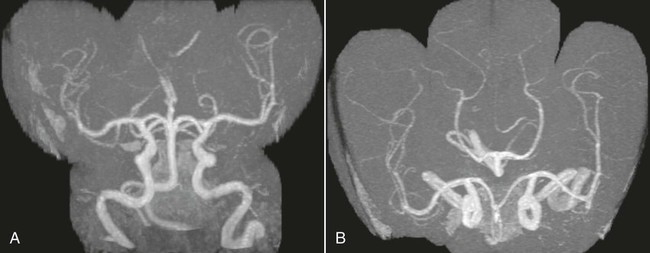
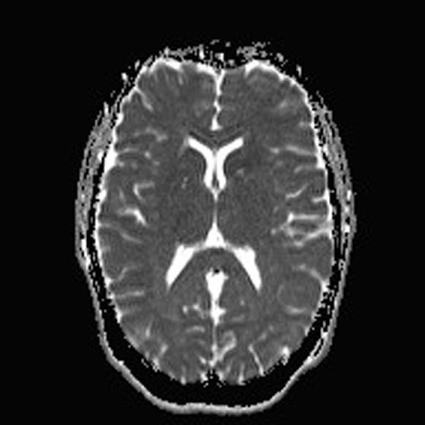
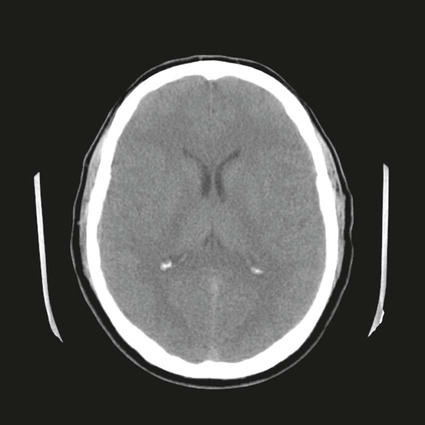
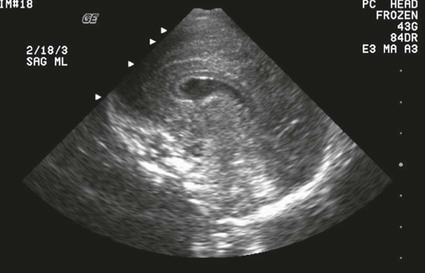
On completion of Chapter 8, the reader should be able to: • Describe the anatomic components of the central nervous system and their general function. • Discuss the roles of the various imaging modalities in evaluation of the central nervous system, particularly magnetic resonance imaging and computed tomography. • Discuss common congenital anomalies of the central nervous system. • Characterize a given condition as inflammatory, degenerative, vascular, or neoplastic. • Identify the pathogenesis of the pathologies cited and typical treatments for them. • Discuss the imaging modalities most commonly used for each type of central nervous system pathology discussed in this chapter. • Describe, in general, the radiographic appearance of each of the given pathologies. The brain consists of the cerebrum (right and left hemispheres), cerebellum, diencephalon (including the hypothalamus), and brainstem. The brainstem, composed of the midbrain, pons, and medulla oblongata, connects the cerebrum with the spinal cord. The innumerable motor and sensory nerves pass through the brainstem into the spinal cord. The spinal cord originates as an extension of the medulla oblongata at the foramen magnum in the base of the skull. It extends to approximately the level of the first or second lumbar vertebra and terminates with a cone-shaped area called the conus medullaris (Fig. 8-1). Spinal nerves beyond this point are referred to as the cauda equina. Both the brain and the spinal cord are covered by the meninges, which consist of three distinct layers (Fig. 8-2). The dura mater is the outermost and is tough and fibrous. It has three major extensions: (1) the falx cerebri, which divides the cerebral hemispheres; (2) the falx cerebelli, which similarly divides the cerebellar hemispheres; and (3) the tentorium cerebelli, which separates the occipital lobe of the cerebrum from the cerebellum. The arachnoid is the middle layer of the meninges and has the appearance of cobwebs. The pia mater is innermost and adheres directly to the cortex of the brain and the spinal cord. The subarachnoid space, at its deepest at the base of the brain, is located between the arachnoid and the pia mater. It is filled with cerebrospinal fluid (CSF) to continuously bathe the brain and the spinal cord with nutrients and to cushion them against shocks and blows. CSF is secreted by the choroid plexus, a network of capillaries located in the brain’s ventricles. The ventricles are four interconnected cavities within the brain. As noted earlier, they house the choroid plexus, which secretes CSF. The right and left lateral ventricles are located in their respective cerebral hemispheres (Fig. 8-3, A and B). They may be further divided into anterior, posterior, and inferior horns, as well as a body and a trigone. CSF flows from the lateral ventricles into the third ventricle via the interventricular foramina (of Monro). The third and fourth ventricles are midline structures connected to each other by the cerebral aqueduct (see Fig. 8-3, C). From there, CSF flows through a median and two lateral foramina (Magendie and Luschka, respectively) into the subarachnoid space surrounding the brain and the spinal cord. Most of the brain’s blood is supplied anteriorly via the bilateral internal carotid arteries and posteriorly via the bilateral vertebral arteries. After entering the cranial vault through the foramen magnum, the vertebral arteries converge to form the basilar artery. The basilar artery and the internal carotid arteries form the circle of Willis (Fig. 8-4) to distribute oxygenated, arterial blood through various branches to all parts of the brain. Venous blood is returned to large venous sinuses in the dura mater, which ultimately drain into the internal jugular veins (Fig. 8-5). Neurons are the primary tissue comprising the nervous system, and they may vary greatly in size. At birth, the human body has an excess of neurons, which begin to die if they are not used. The three basic components of a neuron are the cell body, or soma, which is located within the CNS; dendrites, which carry nerve impulses toward the soma; and axons, responsible for carrying impulses away from the cell body. Most neurons have only one axon, which is covered by a delicate web of connective tissue of Schwann cells covered by a myelin sheath. Myelin is a lipid substance that acts as an insulator and assists in nerve impulse transmission. Neuroglia or supporting cells also play a major role in the nervous system and are much more numerous than neurons. In addition to Schwann cells, neuroglias include astrocytes, oligodendrocytes, ependymal cells, and microglia (Table 8-1). TABLE 8-1 Support Cells of the Nervous System CNS, Central nervous system; PNS, peripheral nervous system. From: McCance, K. L., Huether, S. E.: Pathophysiology: The biologic basis for disease in adults and children, ed 5, St. Louis, MO, 2006, Mosby. Although the intervertebral disks are not part of the CNS, they may come into contact with it when they herniate and impinge on adjacent spinal nerves. Disks cushion the movement of the vertebral column. They are composed of a tough outer covering, known as the annulus fibrosus, and a pulpy center called the nucleus pulposus (Fig. 8-6). Conventional radiographic demonstration of the various cranial structures provides information that is important in evaluation of the CNS. Its role, however, has largely been reduced to evaluation of cranial trauma because of the increased use of magnetic resonance imaging (MRI) and computed tomography (CT). In addition to visualization of fractures caused by trauma, plain skull films may also reveal normal variants. Blood vessels such as the middle meningeal artery commonly cause radiolucent impressions on the inner table of the cranial vault (Fig. 8-7). Their linear progression and bilateral appearance help distinguish them from fractures. Visualization of an enlarged or deformed pituitary fossa provides information about the presence of a pituitary tumor or increased intracranial pressure (ICP) (Fig. 8-8). A calcified pineal gland situated in the midline can be seen on about 60% of all plain skull radiographs (Fig. 8-9). Its displacement may indicate the presence of a pathologic lesion if it is greater than 2 to 3 millimeters (mm). The choroid plexus (Fig. 8-10), falx cerebri (Fig. 8-11), and falx cerebelli may also be calcified. The role of radiography in the evaluation of the spine was described in Chapter 2. A number of conditions that affect the spinal cord can readily be demonstrated (Fig. 8-12). The fluoroscopic procedure of myelography has been a staple of radiology for years, allowing visualization of conditions (such as herniated disks) that impinge on the spinal cord. Its role, however, is diminishing because of the significant specificity of MRI. When myelography is still performed, it is often followed by a CT myelographic examination of the spine. For a wide variety of conditions related to the CNS, MRI is the modality of choice (Fig. 8-13). Its sensitivity is excellent in evaluation of all types of spinal diseases, including tumors, abscesses, and disk disease. The ability of MRI to evaluate brain tumors and conditions such as stroke, cranial tumors, and infection surpasses that of CT. Because MRI does not image the dense petrous bone, it is excellent in evaluating the brainstem and anomalies of the posterior fossa (Fig. 8-14). Evaluation of demyelinating disease such as multiple sclerosis (MS) is substantively enhanced by MRI. It is, however, important to note that the high sensitivity associated with MRI in the diagnosis of myelopathy may also prove to be a limitation at times. The ease with which MRI studies depict expansion and compression of the spinal cord may potentially lead to false-positive examinations and inappropriately aggressive therapies. These issues can be minimized by experienced observers meticulously correlating clinical and radiologic findings. Despite rapid evolution in technology, MRI currently has a small role in the evaluation of trauma, mainly limited to evaluation of spinal cord compression and, in some instances, vertebral fractures. Magnetic resonance angiography (MRA) is used to evaluate the vascular anatomy of the head and neck (Fig. 8-15). It has been proven to accurately locate vascular occlusions within the large arteries of the brain that may lead to a stroke. MRA also demonstrates the major veins and dural sinuses within the brain and plays a large role in the diagnosis and treatment of cerebral venous thrombosis. To date, it has not replaced conventional cerebral angiography but is often used as an adjunct modality. Diffusion-weighted imaging (DWI) involves monitoring the extent to which water can freely move in any volume element (voxel). When the motion of water molecules within a voxel is restricted, greater magnetization will result, and the associated voxels will appear brighter. Reduced water diffusivity has been correlated with more aggressive tumor behavior and is sometimes seen with high cellular density and tumor recurrence as well as ischemic stroke (Fig. 8-16). Diffusion-tensor imaging (DTI) capitalizes on the expected relative increase in restricted water molecules among neurologic fiber tracts by acquiring a three-dimensional map of the fiber tracts with a postprocessing technique referred to as tractography. The higher information content of the voxels makes DTI extremely sensitive to subtle pathology in the brain, which may be preventing the natural movement of water in the brain. Perfusion-weighted imaging (PWI) involves the use of paramagnetic contrast agents to assess the cerebral hemodynamic status of a patient. By utilizing acquisition times that are short enough to capture the changes in signal intensity as a bolus of contrast material passes through the brain, PWI provides useful information on the vascular supply of various tumors, which is only implied from conventional MRI. The relative cerebral blood volume (rCBV) within a tumor can also be measured and compared with the contralateral normal white matter, typically displayed on color maps. The perfusion curves that are generated provide additional prognostic information by distinguishing between the characteristics of benign and malignant tumors. Investigative studies that are now under way indicate that intraarterial MRI perfusion methods could be useful in better understanding the perfusion characteristics of some tumors and may also be useful in monitoring the delivery of therapeutics (Fig. 8-17). CT continues to play a significant role in the evaluation of the CNS. It is rapid, noninvasive, and reasonably accurate. Because CT has excellent contrast resolution, it readily differentiates among the sulci, ventricles, and the gray and white matter within the brain (Fig. 8-18). It is particularly useful in evaluating cerebral bleeding after trauma because it readily reveals the extent of hematomas, if present. In many institutions, it is common practice to routinely perform CT examinations of the head and neck after significant trauma. CT of the brain is also performed to evaluate the ventricles, as in the case of hydrocephalus; to demonstrate cortical atrophy, as in the case of dementia; and to identify neoplasms within the brain, which may or may not create a mass effect or shifting of the midline of the brain. In cases of infarction, edema, abscess, or cyst formation, tissue density is decreased (Fig. 8-19). Tissue density is increased by fresh blood or recent hemorrhage and calcifications within the brain. Other related roles include routine anatomic evaluation such as assessment of shunt (an artificial passageway) functioning and assessment of the bony cerebral and visceral skull. Contrast-enhanced CT of the brain is used to demonstrate anomalies in the cerebral vasculature and to delineate neoplastic growth within the cranial vault. It should be noted, however, that CT-related artifacts that may occur in association with bone at the margins of the posterior fossa often require the use of MRI to optimally evaluate pathology in this region of the brain. As discussed earlier, postmyelographic CT of the spine is also fairly prevalent. CT myelograms demonstrate encroachments of the spinal cord and nerve roots, as does MRI, but bone detail is best visualized with CT. CT is also used to evaluate vertebral fractures, as discussed in Chapter 2. Sonography is useful in evaluating the brains of neonates before closure of the fontanels because the fibrous tissue covering the fontanels provides a ready window into the brain (Fig. 8-20). Sonography readily detects cerebral hemorrhage and hydrocephalus. In premature infants, brain tissue around the ventricles (periventricular germinal matrix) is prone to hemorrhage, often resulting in intraventricular hemorrhage. In addition, premature infants are at an increased risk for periventricular white matter infarction. Portable sonography is a noninvasive method of evaluating cerebral anatomy and can be performed in the neonatal intensive care unit (NICU) to evaluate the status of affected infants. In nuclear medicine, radionuclide brain scans are primarily used to confirm brain death in patients with the appropriate clinical signs, generally after trauma or an intracranial bleed (Fig. 8-21). Technetium-labeled flow agents in combination with single photon emission computed tomography (SPECT) may be used experimentally to assess tumors, areas of stroke and ischemia, and Alzheimer disease. To complement the more traditional anatomic evaluation of other modalities, PET scanning allows imaging of the body’s normal chemical processes and provides physiologic evaluation (Fig. 8-22). In high-risk patients with identified intracranial pathology, PET and SPECT both assist in differentiating tumors from infections. Cerebral angiography is used to demonstrate vessel anatomy within the neck and brain (Fig. 8-23). Because of the increased use of MRA and CT angiography (CTA) vascular studies, conventional angiography is most frequently utilized when an interventional therapeutic procedure is warranted. It is used to diagnose and treat neoplastic lesions, stenosis, aneurysms, arteriovenous malformations (AVMs), and congenital anomalies of the CNS. Cerebral blood flow may be visualized by placing the catheter in the aortic arch or in selected vessels such as the carotid or vertebral arteries. Therapeutic devices such as stents and shunts and therapeutic medications are also placed by the interventionalist via catheters, often eliminating the need for surgical intervention. As mentioned in Chapter 2, spina bifida is a condition in which the bony neural arch that encloses and protects the spinal cord is not completely closed (Fig. 8-24). It most commonly occurs in the lumbar region, and the spinal cord and its meninges may or may not herniate through the resultant opening. Elevated α-fetoprotein (AFP) levels in the mother’s blood and on amniocentesis may allow spina bifida to be diagnosed prenatally. The defect and soft tissue sac are confirmed with fetal sonography. Complications depend on the extent of protrusion and range from treatable to life threatening. Any opening of the sac to the exterior of the body risks meningeal infection, so surgical closure is critical. If only the meninges protrude, the condition is termed a meningocele (Fig. 8-25). These are treated surgically without difficulty and usually have an excellent prognosis. A myelocele is a protrusion of the spinal cord, minus its meningeal coverings, which may also be treatable surgically. A meningomyelocele is the most common and most serious of possible conditions, affecting approximately one in every 800 infants and consisting of a protrusion of both the meninges and the spinal cord into the skin of the back (Figs. 8-26 and 8-27
Central Nervous System
Anatomy and Physiology
Cell Type
Primary Functions
Astrocytes
Form specialized contacts
Provide rapid transport for nutrients and metabolites
Believed to form an essential component of the blood–brain barrier
Appear to be the scar-forming cells of the CNS, which may be the foci for seizures
Appear to work with neurons in processing information and memory storage
Oligodendroglia (oligodendrocytes)
Formation of myelin sheath and neurilemma in the CNS
Schwann cells (neurolemmocytes)
Formation of myelin sheath and neurilemma in the PNS
Microglia
Responsible for clearing cellular debris (phagocytic properties)
Ependymal cells
Serve as a lining for ventricles and choroid plexuses involved in production of cerebrospinal fluid
Imaging Considerations
Radiography
Magnetic Resonance Imaging
Computed Tomography
Sonography
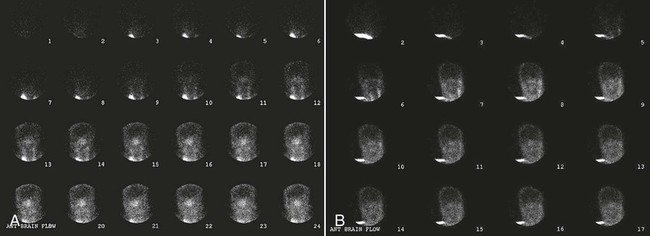
Nuclear Medicine
Vascular and Interventional Radiology
Congenital and Hereditary Diseases
Meningomyelocele (Spina Bifida)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine