Fig. 51.1
Selected (a) axial FLAIR, (b) coronal T1 postcontrast, and (c) sagittal T1 MRI scans of a 31-year-old female medical student who presented with four clinical hemorrhages resulting in headaches, gait instability, and symptoms of hypothalamic dysfunction including hypersomnolence, loss of appetite, and memory difficulties. A cavernous malformation is centered in the midline involving the massa intermedia, hypothalamus, midbrain, third ventricle, and extending to the suprasellar cistern
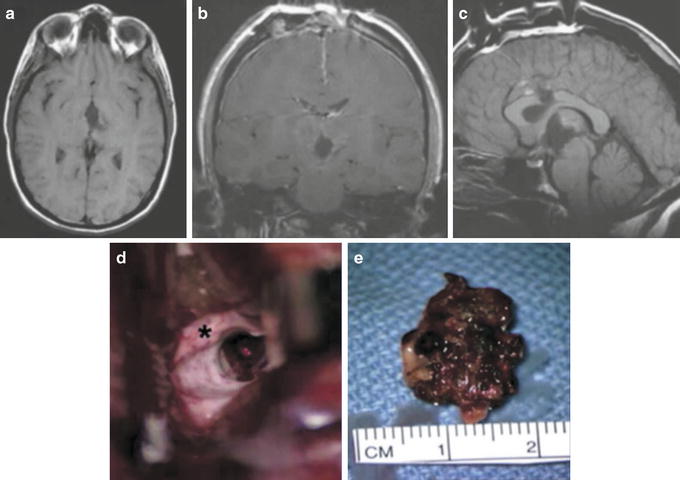
Fig. 51.2
Selected immediately postoperative (a) axial T1, (b) coronal T1 postcontrast, and (c) sagittal T1 MRI scans demonstrate gross total resection of the cavernous malformation seen in Fig. 51.1. (d) Microsurgical removal was performed via an interhemispheric transcallosal approach to the third ventricle. The asterisk is on the corpus callosum, through which a small incision was made that exposed an enlarged foramen of Monro. The laser pointer is focused on the superior aspect of the cavernous malformation. (e) A 3.5-cm cavernous malformation was resected piecemeal from the third ventricle, diencephalon, and midbrain. There was acute, subacute, and chronic hemorrhage within the malformation, which was composed of thin-walled, fragile vascular channels. No attempt was made to remove the surrounding hemosiderin-stained brain. Postoperatively, the patient quickly recovered to her neurologic baseline and has continued to improve, though some memory difficulties still remain

Fig. 51.3
(a) Sagittal T1, (b) coronal T1 postcontrast, and (c) axial T2 and (d) T1 MRI scans illustrate a 1-cm, right midpontine cavernous malformation with subacute hemorrhage surrounded by a rim of hemosiderin and surrounding brainstem edema (arrow in c). Although this lesion did not present to a pial or ventricular surface, three clinically significant hemorrhages were observed over a 7-month period, resulting in diplopia, facial weakness, sensory loss, and mild hemiparesis. Microsurgical resection of the cavernous malformation was therefore performed. (e) A right subtemporal approach was used as illustrated, aided by the intraoperative surgical navigation. Brainstem functional mapping was employed prior to making the few-millimeter incision in the lateral surface of the pons (right upper panel, e). Postoperatively, the patient’s hemiparesis transiently worsened, but he recovered within 1 week and currently has no focal deficits
Treatment
Given the excellent results of microsurgical resection of cavernous malformations, it is generally easy to endorse the philosophy of operative management for most of these lesions. However, increasing evidence suggesting a relatively benign natural history of some lesions has indicated the need for reevaluation of management decisions. In particular, when dealing with a patient harboring several lesions, a family affected by a hereditary form of this condition, or deep-seated lesions in the brainstem, it becomes apparent that an aggressive approach is not always advisable. The only clear indications for surgery are for medically intractable epilepsy, documented recurrent hemorrhage, and progressive neurological deficit. Patients with an established diagnosis of CCM who present without clinical hemorrhage, seizures, or other specific symptoms are usually candidates for clinical observation and repeat imaging. Conservative management is also appropriate for many patients with purely incidental lesions or malformations located deep within functional areas that do not present to a pial or ventricular surface.
Microsurgical Treatment of Supratentorial Lesions
Surgical resection of most cavernous malformations is straightforward because these lesions have no major arterial supply and do not bleed much at surgery. The most difficult task in resection is often intraoperative localization and choosing an appropriate surgical corridor. This has been facilitated with the use of stereotaxic MRI computer-guidance systems for neuronavigation, which allows planning of surgical trajectories to avoid functional cortex and major blood vessels, as well as target localization to within 1–2 mm. The risk of open microsurgery is generally related to lesion size, location, and proximity to critical structures. In addition, advanced age, medical comorbidities, and poor neurological condition are all negative prognostic factors for a good surgical result. Several surgical series have reported excellent results in removal of superficial lesions of both eloquent and noneloquent areas. More than 75 % of all cavernous malformations are located supratentorially [22], most of which are located in cortical and subcortical regions that are readily accessible with modern surgical techniques. Even prior to MRI, surgical series have documented the successful removal of cavernous malformations with low risk. Vaquero et al. report successful treatment of 19 supratentorial cavernous malformations located in the cerebral hemispheres from 1977 to 1987. Of the 19 patients, 17 had excellent results (no seizures without medication) and two were classified as good outcomes (occasional seizures with medication) [70]. There were no poor outcomes or deaths in this cohort. Similarly, excellent results have been documented in the post-MRI era. McCormick reviewed his experience with removal of supratentorial cavernous malformations. In 63 of 65 patients, complete removal was obtained, two patients experienced worsening of an existing deficit, a third patient developed a new deficit, and there was only one postoperative death [71]. When performed for medically intractable epilepsy, several published results confirm a greater than 90 % likelihood of a seizure-free outcome with surgical extirpation of cavernous malformations and the surrounding epileptogenic tissue [69].
The surgical approach is defined by the lesion location, and in general the craniotomy is centered over the lesion. The use of neuronavigation allows precise targeting that minimizes the size of the craniotomy and risk to exposed brain (Fig. 51.3). Lesions that involve the anterior corpus callosum near the genu and cingulate gyrus can be approached through a small frontal craniotomy. Lesions that are more basal in the subfrontal or septal regions often require a lower frontal craniotomy through a bicoronal incision or an interhemispheric approach. Posterior parasagittal approaches are generally used for lesions in the body of the corpus callosum, cingulate gyrus, and midline posterior frontal, parietal, or occipital areas (Fig. 51.4), as well as for some thalamic CMs. Medial anterior temporal lesions involving the amygdala, the hippocampus, or the uncus are approached through the Sylvian fissure via a standard pterional craniotomy. Superficial cortical lesions are often easy to identify because of overlying cortical discoloration, whereas small subcortical lesions often require stereotaxis. Both trans-sulcal and trans-gyral approaches can be employed depending on lesion geometry and the location of cortical vasculature. Once identified, a well-defined gliotic plane typically allows for easy separation of the lesion from surrounding tissue so that complete lesion resection can be achieved. When surgery is indicated for seizures, the hemosiderin- or hematoidin-laden gliotic tissue should be resected due to its role in seizure generation. Intraoperative electrocorticography may be helpful for confirming the extent of abnormal electrical activity, particularly for lesions near the motor or sensory cortices. For dominant hemisphere lesions, awake intraoperative speech mapping may be required to preserve language function.


Fig. 51.4
Selected 3-year postoperative (a) sagittal, (b) coronal, and (c) axial T1 MRI scans demonstrate gross total resection of the cavernous malformation seen in Fig. 51.3
Microsurgical Treatment of Infratentorial Lesions
Lesions located deep within the brain are difficult to remove and present special challenges (Figs. 51.1, 51.2, 51.3, and 51.4), though recent series describe improved outcomes with image-guided techniques. Approximately 10–30 % of all intracranial cavernous malformations are located in the posterior fossa [21–25], and some reports suggest these lesions may be more frequently symptomatic [21]. Surgical removal is indicated for symptomatic lesions located in the cerebellum or superficially in the brainstem when eloquent parenchyma can be spared. Several surgical approaches have been detailed in the literature including suboccipital, infratentorial supracerebellar, occipital transtentorial, far lateral, transtemporal, subtemporal transtentorial, and combined petrosal. Furthermore, much emphasis has been placed on the selection of an approach that optimizes exposure to the lesion with minimum disruption to surrounding structures. In general, lesions of the cerebellar vermis, medial cerebellar hemispheres, floor of the fourth ventricle, and dorsal medulla are easily approached through a standard suboccipital approach. Lesions of the tectum and pineal region are often best approached via infratentorial supracerebellar access. The occipital transtentorial approach provides exposure of the superior cerebellar peduncles and vermis, the anterior medullary velum, posterior third ventricle, the splenium, and the quadrigeminal plate. The far lateral approach provides excellent exposure of the anterior and lateral medulla and cervicomedullary junction. Transtemporal approaches allow access to lesions in and around the internal auditory meatus such as pontomedullary junction cavernous malformations and can be employed in patients without serviceable hearing. Cavernous malformations involving the anterior midbrain–interpeduncular fossa region can be approached through a pterional trans-sylvian, subtemporal, or orbitozygomatic craniotomy, and occasionally lesions located in the upper two-thirds of the brainstem require a combined petrosal approach. CSF drainage for brain relaxation, electrophysiological monitoring, and functional mapping are all important for minimizing complications and reducing the risk of permanent neurological deficit.
Unlike supratentorial cavernous malformations, deep lesions can be extremely adherent to the normal parenchyma and are sometimes removed in a piecemeal fashion following circumferential separation and devascularization from the surrounding gliosis. All perforating arterial vessels must be dissected meticulously and preserved. Furthermore, the hemosiderin staining surrounding the malformation should be spared from surgical removal. An association between cavernous malformations and venous malformation has been described and may be as high as 16 % [72], and while the goal of surgery is complete excision of the cavernoma, interruption of a venous malformation is to be avoided because of the risk of venous infarction. The risk of open microsurgery for superficial cerebellar lesions is low, similar to that for most supratentorial cavernous malformations. The risks associated with surgical exploration of deep-brain and brainstem cavernous malformations is considerably higher, as discussed below.
Radiosurgical Treatment of Cavernous Malformations
A number of groups have used stereotactic-focused radiosurgery with heavy-charged particles (protons or helium ions) or photons (Gamma Knife or LINAC radiosurgery) in attempts to improve the natural history of deep, difficult-to-resect cavernous malformations [13, 73–81]. Although there has been no prospective randomized study, recent reports from several groups suggest that, for certain patients, radiosurgery may decrease the risk of clinical hemorrhage, compared with the statistically derived natural history [13, 73, 74]. Our combined Stanford University–University of California, Berkeley, program previously treated 57 cavernous malformations in the brainstem, thalamus, and basal ganglia using Bragg peak helium ion radiosurgery (47 lesions) or LINAC radiosurgery (10 lesions), with mixed results [74]. Eighteen patients (32 %) experienced symptomatic bleeding (20 hemorrhaging episodes) after radiosurgery. Sixteen hemorrhaging episodes occurred within 36 months after radiosurgery (9.4 % annual bleeding rate; 16 hemorrhaging episodes/171 patient-years), and 4 hemorrhaging episodes occurred more than 36 months after treatment (1.6 % annual bleeding rate; 4 hemorrhaging episodes/257 patient-years) (P < 0.001). The radiosurgery group at Harvard University reported a decrease in the annual hemorrhage rate from 17.3 % before radiosurgery to 4.5 % after a latency period of 2 years after radiosurgery [73]. Similar symptomatic hemorrhage rates after Gamma Knife radiosurgery for cavernous malformations were reduced from 8.8 % during the first 2 years after radiosurgery to 1.1 % thereafter [13]. A more recent study of 103 patients with solitary cavernous malformations who were treated with Gamma Knife radiosurgery at the University of Pittsburgh with follow-up from 2 to 20 years demonstrated a 10.8 % annual hemorrhage rate for the first 2 years after treatment and then a 1.06 % annual bleed rate after 2 years [82]. However, statistical modeling analysis showed that the hemorrhage rate from untreated cavernous malformations appeared to demonstrate temporal clustering of hemorrhages such that the rehemorrhage rate from untreated cavernous malformations was found to be high initially (2 % monthly rehemorrhage rate during the first 2.5 years following hemorrhage; 14 % cumulative incidence of a second hemorrhage during the first year) with decreases 2–3 years after a previous hemorrhage (to less than 1 % per month) [62]. These results therefore question whether the data from the radiosurgical series represent protective effects of treatment or simply the natural bleeding history of these lesions. Additionally, in ten patients who underwent microsurgical resection of their cavernous malformations 1–10 years following radiosurgical treatment, none of the cavernous malformations were found to be thromobosed on pathological examination [83]. Furthermore, in our series of patients with cavernous malformations who underwent radiosurgery, 7 % experienced symptomatic radiation edema and 2 % experienced radiation necrosis [74] (Fig. 51.5). Other groups have documented a 13.5 % incidence of new neurologic deficits [82] and a 16 % incidence of permanent neurological deficits with a 3 % mortality rate secondary to radiation-induced complications [73].


Fig. 51.5
(a) Axial proton-density MRI of a 35-year-old woman who initially presented with generalized seizures shows a 1-cm vascular malformation located in the left thalamus adjacent to the posterior limb of the internal capsule. She was first treated with stereotactic heavy-charged-particle Bragg peak radiosurgery. Nine months after radiosurgery, she experienced worsening right hemiparesis; (b) axial T2 imaging at that time demonstrated extensive radiation-induced changes extending into the basal ganglia and thalamus. She subsequently underwent stereotactically guided microsurgical resection via a small left posterior parietal, interhemispheric transcallosal approach through the pulvinar. The patient recovered extremely well and was discharged 2 days after surgery without new deficits. (c) Hematoxylin and eosin-stained specimen from a surgically removed brainstem cavernous malformation 5 years after stereotactic heavy-charged-particle radiosurgery. Patent vascular channels (arrow) are observed. (d) Surgical specimen from tissue adjacent to a cavernous malformation removed from another patient with delayed radiation-induced injury 3 years after stereotactic radiosurgery of a right parietal cavernous malformation. Histopathologic changes consisted of thick-walled hyalinized vessels with fibrinoid necrosis (arrow), postradiation gliosis, and reactive astrocytosis and necrosis (arrowhead), characteristic of radiation effects
Brainstem Cavernous Malformations
Brainstem cavernomas, though very rare, deserve special mention because their clinical presentation, natural history, and treatment decisions often differ from those of cavernous malformations in other locations. Such lesions account for 9–35 % of all cavernomas and for 18–22 % of all intracranial cavernomas [21, 23, 25]. Fifty to 70 % of these lesions occur in the pons, 20–35 % in the midbrain, and 15–25 % in the medulla. Symptoms vary from cranial nerve deficits, sensory dysfunction, paresis/plegia, ataxia, dysmetria, speech difficulty to headaches and decreased levels of consciousness. The clinical presentation can often be confusing and many patients who have experienced episodic clinical deterioration secondary to hemorrhaging have been diagnosed with having multiple sclerosis.
There is evidence that the natural history of brainstem cavernous malformations differs from that of other locations, suggesting that this subgroup has a higher propensity for clinical bleeding. Porter et al. reported nearly all of their 100 brainstem cavernomas presented with evidence of clinically significant hemorrhage, 56 % with multiple bleeds, and 22 % with more than two clinical bleeds [21]. While some patients with hemorrhage from brainstem cavernous malformations make good recoveries without intervention, it is also clear that multiple bleeding episodes increase the likelihood of a persistent deficit. Various series have demonstrated recurrent hemorrhage rates of 5–60 % [21, 66, 84–87]. Furthermore, when multiple bleeding episodes do occur, the time interval between hemorrhages is increased and the mortality rate for each hemorrhage may be as high as 20 %.
Surgical treatment of brainstem cavernous malformations is considerably more risky than operative intervention in other less eloquent areas of the nervous system. Difficult access, narrow surgical corridors, and sensitivity to injury of critical structures near these lesions present unique obstacles. Despite the high risk of permanent morbidity and technical challenges of microsurgical excision, several centers have demonstrated expertise in removing these lesions with good outcomes. Pandey et al. reported a series of 176 patients with 179 deep-brain cavernous malformations located in the brainstem (n = 136), thalamus (n = 16), and basal ganglia (n = 26), which were microsurgically resected [86]. Neuronavigation, mild hypothermia (32–33 °C), electrophysiological monitoring, cranial nerve mapping, and use of the CO2 laser assisted in the safe removal of these lesions. Over a 20-year period with a mean follow-up of 3.5 years, the overall results were excellent (mRS = 0–1) in 54 %, good (mRS = 2) in 29 %, and poor (mRS = 3–6) in 18 %; 5 % of patients died. Ninety-six percent of these lesions were completely resected after a single surgery (86 %) or 2 surgeries (9.6 %), 88 % of the patients were either neurologically improved or unchanged after 6 months, and only 12 % of the patients were worse than their preoperative status at the last follow-up. Of 135 patients undergoing microsurgical resection of 136 brainstem cavernous malformations in this series, although slightly less than 1/3 of the patients were immediately worse after surgery, at long-term follow-up (>6 months), 87 % of the patients were neurologically unchanged or improved, while 13 % had worsened. Porter et al. reported their experience with 100 patients with brainstem cavernous malformations, of which 86 underwent microsurgical resection. Over a 12-year period, 87 % were the same or better, 10 % were worse, and 4 % died; the long-term neurological morbidity rate was 12 %. Bertalanffy et al. reviewed their series of 24 brainstem cavernous malformations over a 5-year period [23]. Seventy percent had Karnofsky scores of 90 or better at follow-up, 21 % had scores of 60–70, and 4 % had scores less than 60. Their rate of long-term morbidity was 5.5 % and there were no deaths. Therefore, while the risks of surgical intervention for brainstem and deep-brain cavernous malformations are not low, the natural history of such lesions is likely worse than that of cavernous malformations at other CNS sites, and we recommend that surgical resection be considered for symptomatic lesions that present to a pial or ependymal surface and in certain circumstances even for those malformations that do not present to a pial/ependymal surface, if they have bled repetitively and can be exposed through a safe corridor [86].
Conclusions
Surgical resection of symptomatic cavernous malformations provides an excellent treatment option and, when completely excised, is curative for most lesions. When cavernous malformations are removed in patients with medically refractory epilepsy, improved seizure control is typical and many centers report high rates of “seizure-free” patients. In general, the surgical morbidity associated with the resection of superficial lesions in the cerebral and cerebellar cortices is minimal, and mortality is considered unusual. Resection of lesions in and around eloquent cortex requires electrophysiological monitoring and often functional mapping, but can be performed safely. Deep-seated cavernous malformations in the thalamus, basal ganglia, and brainstem are associated with higher surgical risks; however, for symptomatic lesions that demonstrate recurrent hemorrhage and present to a pial or ventricular surface, resection by an experienced neurosurgeon is well reported and should be considered. The role of radiosurgery for unresectable malformations remains uncertain and at our institution was associated with significant risks of radiation-induced injury without any significant change in the natural history of hemorrhaging. Until better evidence of its efficacy is established, we do not recommend radiosurgery for treatment of cavernous malformations.
Acknowledgement
This work was supported in part by support from Bernard and Ronni Lacroute, the William Randolph Hearst Foundation, and Russell and Beth Siegelman (to GKS).
References
1.
Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003; 34(5):1163–9.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree