Catherine M. Owens, Carolyn Young, Øystein E. Olsen
Challenges and Overview of Special Features and Techniques
Ultrasound is the primary diagnostic technique for follow-up in paediatric imaging, and its application is described in the organ-specific chapters in this section. This chapter describes general principles for the application of radiography, computed tomography and magnetic resonance imaging in paediatric radiology.
Medical diagnostic imaging has evolved and rapidly improved over the past five decades as a result of novel development in diagnostic digital imaging and interventional techniques. With technical advances in computer processing power, along with high-resolution display monitors/workstations, together with increased computing power and electronic data archive systems, diagnostic imaging departments have transformed, from being labour-intensive analogue film-based imaging units into fully integrated digital environments.
However, with all this new technology readily available, there remains a lack of dedicated purpose-built equipment suitable for use in children and easily available on the market.
Although manufacturers are aware of the more important radiation implications pertaining to children, and have made inroads into lowering medical radiation doses, the ultimate responsibility remains with radiology technicians and radiologists, who control and operate diagnostic equipment, to adapt and adjust the (primarily adult-designed) techniques and protocols to suit the younger, more radiation-vulnerable population.
Projection Radiography
The development of digital imaging in plain film radiography is advantageous within paediatric imaging. First introduced to computed radiography (CR) and later in direct readout radiography (DR) systems (utilising flat-panel detector (FD) technology), this technology helped provide greater efficiency in converting incident X-ray energy into image signal. This, together with its inherent wide dynamic range, greatly improved image quality, when compared with conventional screen-film-based systems, if equivalent exposure parameters were used. This has the potential for lowering radiation dosage for the patient and also reducing the risk of failed, i.e. non-diagnostic, exposure. Using post-processing capabilities, both bone and soft-tissue anatomy can now be optimally displayed on the same image, thus eliminating the need for repeated radiation exposure.1–3
However, care must be taken when setting exposure factors, as unlike with film-based techniques, overexposure can easily occur with digital imaging. This happens without adverse effect on image quality, and may not be recognised by the operator, as the image brightness can be freely adjusted, independent of exposure level.4,5
In general, FD technology is an efficient method for obtaining high-quality image data and enabling immediate image preview, storage and distribution over local area networks for viewing by clinicians, thus enhancing efficiency and productivity within high workflow departments.
Other applications of FD technology include digital tomosynthesis (or digital tomography), providing quasi 3-dimensional images, adapted for use in chest imaging. As a chest radiograph is a 2-dimensional image, sensitivity may be reduced when detecting underlying pathology because of overlapping anatomy. This can be overcome by CT applications but with an inherent increased radiation dose. Tomosynthesis evolved from conventional geometric tomography and was introduced as a low-dose alternative for chest radiographic examination in monitoring children with cystic fibrosis, and in the detection of pulmonary nodules.6,7 This technique, involving the acquisition of a number of projection images at different angles during a single vertical motion of the X-ray tube (between a given angular range of −17.5° and +17.5°) directed at a stationary digital flat-panel detector, results in up to 60 coronal sectional images at an arbitrary depth.6–8 Anatomical structures within each image section are sharply depicted, whilst structures located anteriorly and posteriorly are blurred. Spatial resolution is higher in tomosynthesis than in CT in the acquired imaging plane, but depth resolution is inferior, due to the limited angles used.
Further limitations to this imaging technique include the necessity of a 10-s acquisition time, increasing the likelihood of respiratory motion artefacts in non-compliant patients, which will exclude younger children who are unable to hold their breath. Although the radiation dose for tomosynthesis is much reduced compared with CT, it is three times higher than that for a frontal chest radiograph. This can be offset by a higher nodular detection rate than that seen with the plain radiograph, making this technique a possible alternative to CT examinations in some children.
Fluoroscopy
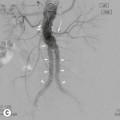
The introduction of digital fluoroscopy with its high-speed digitisation of the analogue video signal has revolutionised real-time fluoroscopy that relied on the use of image intensifier/TV systems to display the diagnostic image. Development of fluoroscopy FD technology with its fast digital readout and dynamic acquisition of image series at high frame rates (up to 60 frames per second) has become a well-established application in cardiac paediatric angiography. The other important application is within minimally invasive interventional procedures, due to their less invasive nature, when compared with conventional surgery. Advantages of FD compared with image intensifier systems that help minimise radiation dose include pulsed fluoroscopy, last-image hold and screen capture, which negate further diagnostic image exposures. Other features that improve image quality include homogeneous image uniformity with lack of geometric distortion across the entire image, reduced veiling glare, and a rectangular or square field-of-view, which utilises the full width of the image monitor. The small compact size of FD mounted on a dedicated C-arm system increases operational flexibility and ease of patient access, both features which are particularly pertinent within paediatric imaging.2,3
Modern C-arm angiography systems utilising FD technology are equipped with rotational angiography applications, providing 3-dimensional CT image capture (FD-CT) that is used mainly in interventional procedures. The ability to combine 2-dimensional fluoroscopic and 3-dimensional CT imaging within a single unit is advantageous for providing planning, guidance and monitoring of interventional procedures and intraoperative imaging.9,10 The image quality is lower in FD-CT than in clinical CT, but in situations where a quick CT control diagnosis is required, an alternative lower spatial resolution image is acceptable. In addition, due to the slow rotation of FD-CT, patient movement and respiratory artefacts in body imaging further reduce spatial resolution. The radiation dose is noted to be higher in FD-CT due to lower detection efficiency, although the milliamperes per second (mAs) per single image acquisition is much lower. It is the cumulative dose of the procedure that is crucial in this instance, with variation seen in each individual investigation/treatment.
Computed Tomography (CT)
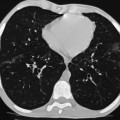
CT is a proven essential diagnostic imaging technique and is considered the most sensitive method for evaluating airway diseases in children.
Two CT imaging configurations exist: namely, multidetector CT (MDCT) with up to 320 detector rows and dual-source CT (DSCT) utilising MDCT technology. The increasing temporal and better spatial resolutions have extended the role of CT applications in young children to include cardiac imaging. Advantages of these systems include subsecond tube rotation times (down to 0.33 s). This increase in acquisition speed has the potential for reducing motion and respiratory artefacts and improving image quality. The overall reduction in examination acquisition time may also obviate the need for sedation in some children. The availability of small detector elements (0.5 mm) combined with thin-slice collimation provides isotropic resolution that allows image data to be manipulated/reformatted in any orthogonal plane and displayed as either 2- or 3-dimensional images that have the same spatial resolution as the base axial data set and with reduced partial volume artefact.
320-Row MDCT
The availability of 320-slice MDCT allows for larger volume coverage of up to 16 cm in the z-axis coverage. The advantage is that this coverage is well within the clinical range of thoracic length in neonates and young children. Therefore, imaging of the entire chest can be accomplished in a single-volume cone beam acquisition during one tube rotation of 0.35 s.11 This is much faster than either helical MDCT or DSCT acquisition. Axial volumetric acquisitions have the potential of radiation dose saving. Due to the large nominal beam width used, the contribution of the penumbra effect is less prominent. Also, unlike in helical CT, over-ranging in the longitudinal axis is not applicable in this instance, as the exposed range corresponds exactly to the imaged range and therefore more effective usage of the radiation exposure for image formation. Axial volumetric acquisition can be applied to other clinical situations that include cardiac imaging in children, as when using prospective ECG-gating, the entire heart can be imaged within a single tube rotation.11
Dual-Source CT
The second-generation DSCT system (Siemens Flash, Forchheim, Germany) is currently the latest in CT technology. It incorporates two X-ray tubes each with corresponding 64-row detector systems (each contributing 128 slices by means of a z flying focal spot), mounted at an angular offset of 90° to each other. Designed for cardiac imaging, the two-tube detector system does not operate simultaneously, but in tandem, whereby data from the second detector system are collected a quarter of a rotation later following the first set of detectors. This allows gapless volume high-pitch CT (up to 3.2 pitch), avoiding overlapping slices with reduced radiation dose.12,13 Together with a fast gantry rotation time of 0.28 s, a 75-ms temporal resolution is achieved, enabling helical prospective ECG-triggered cardiac imaging for the first time.14 High heart rates are no longer a limiting factor when imaging children, and DSCT is invaluable for both the pre- and post-surgical assessment of a wide variety of congenital heart diseases, resulting in improved visualisation of the coronary arteries if data are captured in the systolic phase15 even in younger children. Prospectively gated cardiac imaging is the preferred technique in young children, where often only morphological and proximal coronary artery detail is required. This negates the need for retrospectively gated imaging with its higher radiation burden. The sharp anatomical delineation between adjacent structures seen in prospective gating is superior to that seen in non-gated CTA studies, and with lower radiation dose levels.
Dual-Energy Dual-Source CT
The availability of two X-ray tubes allows simultaneous acquisition of two data sets at different tube potentials (80 and 140 kVp), during the same phase of contrast enhancement and excludes temporal changes and spatial misregistration. This technique takes advantage of differences between tissue and material composition and differences in their photon absorption characteristics. In particular, materials with a high atomic number (like iodine) exhibit a different degree of attenuation between the two tube potentials. By applying specific post-processing algorithms to the acquired data, virtual unenhanced and virtual angiographic data sets can be generated, based on the three-material composition principle;16–18 e.g. within the abdomen, the materials analysed are soft tissue, fat and iodine, whilst in the chest, soft tissue, air and iodine are analysed. Application of the bone removal algorithm will display an angiographic data set without overlying bony structures, resulting in easier image interpretation. This eliminates the need for pre-intravenous contrast CT examinations imaging, as may be required if using a single tube device, for data subtraction purposes. Thus radiation doses are halved.
Overall radiation dose associated with dual-energy CT (DECT) is noted to be comparable to that of single-source MDCT systems. Other clinical dual-energy applications include characterisation of abdominal masses, chemical composition of renal calculi, myocardial and pulmonary perfusion imaging.
The depiction of iodine distribution for the detection of peripheral lung perfusion defects from suspected pulmonary emboli adds functional information to conventional pulmonary CT angiography. Its application in paediatrics is relevant in the evaluation of subsegmental pulmonary emboli.17,19 By applying advanced post-processing software to the acquired data set, an iodine distribution map is generated and overlaid onto a grey-scale image. A normal perfusion image will show homogeneous colour distribution extending to the lung periphery and is displayed in a multiplanar format that can be manipulated manually. The presence of a filling or hypoperfusion is indicative of an obstructed vessel supplying the relevant lung segment. Review of the grey-scale image will help determine anatomical detail and location.
Other DECT applications include the use of xenon ventilation in chest imaging, as it is found to be more sensitive in the evaluation of regional and global airway and lung abnormalities in children.20 Conventional CT investigation requires both inspiratory and expiratory acquisitions to demonstrate whether air-trapping exists. However, the use of xenon with the ability to demonstrate regional ventilation defects on inspiration obviates the need for an additional expiratory phase acquisition, with consequent radiation dose saving. In addition, the quantitative evaluation of lung density on CT is dependent on age and level of respiration. As this varies in young children, the use of xenon with its insensitivity to respiration level will provide more accurate measurements.
Radiation Dose Consideration
The increase in radiation burden associated with CT imaging and the potential risk to children cannot be ignored. CT requests must, therefore, be justified; a robust risk–benefit analysis should always be carried out before undertaking CT examination in children. Imaging techniques and dedicated paediatric protocols must be available to the operators, ensuring adherence to the ALARA principle. Adjustments to the CT parameters could be based on the patient’s age, body weight or body diameter.
A list of guidelines for routine imaging are detailed in Tables 75-1 to 75-5. The use of 120 kVp is no longer advocated in paediatric body imaging, and can be reduced to 80 or 100 kVp21 without detriment to image quality, and, indeed, improves contrast resolution, thus allowing a smaller concentration of contrast medium to be administered. The tube current can also be reduced but at the detriment of increased image noise and reduced spatial resolution.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree