CHAPTER THREE Chest
The chest radiograph is one of the most commonly obtained examinations in pediatric imaging. It is also the examination most likely to be encountered by radiology residents, pediatric residents, general radiologists, and pediatricians. Therefore, topics such as chest imaging in neonates and the evaluation of suspected pneumonia are discussed in detail.
NEONATAL CHEST
Diffuse Pulmonary Disease in the Newborn
Lung volumes can be categorized as high, normal, or low. Normally, the apex of the dome of the diaphragm is expected to be at the level of approximately the tenth posterior rib. Lung opacity, if present, can be characterized as streaky, perihilar (central) densities that have a linear quality or as diffuse, granular opacities that have an almost sandlike character. Classically, cases fall into one of the following two categories: (1) cases with high lung volumes and streaky perihilar densities and (2) cases with low lung volumes and granular opacities (Table 3-1). This is more of a guideline, rather than a rule, because many neonates with diffuse pulmonary disease have normal lung volumes. The differential diagnosis for cases with high lung volumes and streaky perihilar densities includes meconium aspiration, transient tachypnea of the newborn, and neonatal pneumonia. Most of the neonates in this group are term. The differential for cases with low lung volumes and granular opacities includes surfactant deficiency and β-hemolytic streptococcal pneumonia. Most of these neonates are premature.
TABLE 3-1. Differential Diagnosis of Diffuse Pulmonary Disease in the Newborn
| High lung volumes, streaky perihilar densities | Low lung volumes, granular opacities |
|---|---|
Meconium Aspiration Syndrome
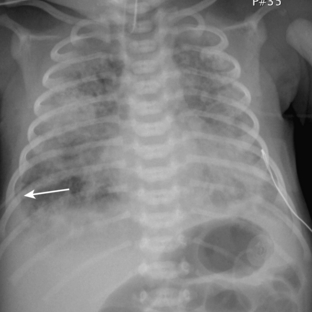
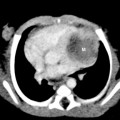
Meconium aspiration syndrome results from intrapartum or intrauterine aspiration of meconium. It usually occurs secondary to stress, such as hypoxia, and more often occurs in term or postmature neonates. The aspirated meconium causes both obstruction of small airways secondary to its tenacious nature and also chemical pneumonitis. The degree of respiratory failure can be severe. Radiographic findings include hyperinflation (high lung volumes), which may be asymmetric and patchy, and asymmetric lung densities that tend to have a ropy appearance and a perihilar distribution (Fig. 3-1). Commonly there are areas of hyperinflation alternating with areas of atelectasis. Pleural effusions can be present. Because of the small-airway obstruction by the meconium, air-block complications are common, with pneumothorax occurring in 20% to 40% of cases. Meconium aspiration syndrome is relatively common; 25,000 to 30,000 cases occur in the United States annually.
Transient Tachypnea of the Newborn
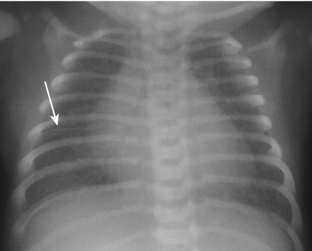
Transient tachypnea of the newborn (TTN) is also referred to by a variety of other names, including wet lung disease and transient respiratory distress. It occurs secondary to delayed clearance of fetal lung fluid. Physiologically, the clearing of fetal lung fluid is facilitated by the “thoracic squeeze” during vaginal deliveries; therefore, most cases of TTN are related to cesarean section in which the thoracic squeeze is bypassed. Other causes include maternal diabetes and maternal sedation. The hallmark of TTN is a benign course. Respiratory distress develops by 6 hours of age, peaks at 1 day of age, and is resolved by 2 to 3 days. There is a spectrum of radiographic findings similar to those seen with mild to severe pulmonary edema. There is a combination of airspace opacification, coarse interstitial markings, prominent and indistinct pulmonary vasculature, fluid in the fissures, pleural effusion, and cardiomegaly (Fig. 3-2). Lung volumes are normal to increased.
Neonatal Pneumonia
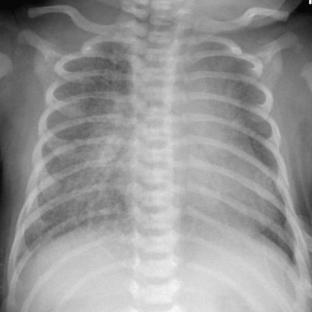
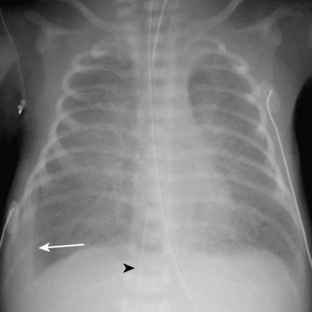
Neonatal pneumonia can be caused by a large number of infectious agents that can be acquired intrauterine, during birth, or soon after birth. With the exception of β-hemolytic streptococcal pneumonia, which will be discussed separately, the radiographic appearance of neonatal pneumonia is that of patchy, asymmetric perihilar densities and hyperinflation (Fig. 3-3). Pleural effusions may be present (Fig. 3-4). Such cases of neonatal pneumonia may have a similar radiographic appearance to and be indistinguishable from meconium aspiration syndrome when using imaging alone.
Surfactant-Deficient Disease
Surfactant-deficient disease (SDD; also referred to as respiratory distress syndrome or hyaline membrane disease) is a common disorder, with approximately 40,000 new cases annually in the United States. It is primarily a disease of premature infants, affecting up to 50% of them, and it is the most common cause of death in live newborns. SDD is related to the inability of premature type II pneumocytes to produce surfactant. Normally, surfactant coats the alveolar surfaces and decreases surface tension, allowing for the alveoli to remain open. As a result of the lack of surfactant, there is alveolar collapse, resulting in noncompliant lungs. The radiographic findings reflect these pathologic changes (Fig. 3-5A, B). Lung volumes are low. There are bilateral granular opacities that represent collapsed alveoli interspersed with open alveoli. Because the larger bronchi do not collapse, there are prominent air bronchograms. When the process is severe enough and the majority of alveoli are collapsed, there may be coalescence of the granular opacities, resulting in diffuse lung opacity. A normal film at 6 hours of age excludes the presence of SDD.
Surfactant Replacement Therapy
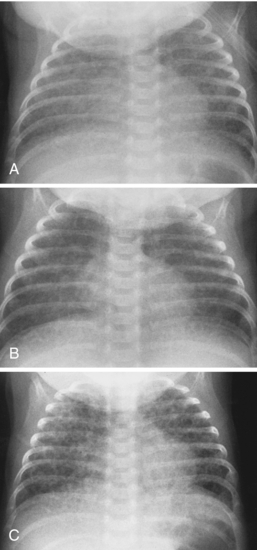
One of the therapies for SDD is surfactant administration. Surfactant can be administered via nebulized or aerosol forms. It is administered into the trachea via a catheter or an adapted endotracheal tube. The administration of surfactant in neonates with SDD has been shown to be associated with decreased oxygen and ventilator setting requirements, decreased air-block complications, decreased incidence of intracranial hemorrhage and bronchopulmonary dysplasia, and decreased death rate. However, there is an associated increased risk for development of patent ductus arteriosus and pulmonary hemorrhage, and there can be an acute desaturation episode in response to surfactant administration. Surfactant administration can be given on a rescue basis when premature neonates develop respiratory distress or can be given prophylactically in premature infants who are at risk. Prophylactic administration is commonly given immediately after birth and is becoming a more common practice. In response to surfactant administration, radiography may demonstrate complete, central, or asymmetric clearing of the findings of SDD (see Fig. 3-5). There is usually an increase in lung volumes. Neonates without radiographic findings of a response to surfactant have poorer prognoses than those who have radiographic evidence of a response. A pattern of alternating distended and collapsed acini may create a radiographic pattern of bubblelike lucencies that can mimic pulmonary interstitial emphysema. Knowledge of when surfactant has been administered is helpful in rendering accurate interpretation of chest radiographs taken in the neonatal intensive care unit (NICU).
β-Hemolytic Streptococcal Pneumonia
β-hemolytic (group B) streptococcal pneumonia is the most common type of pneumonia in neonates. The infection is acquired during birth, and at least 25% of women in labor are colonized by the organism. Premature infants are more commonly infected than are term infants. In contrast to the other types of neonatal pneumonias, the radiographic findings include bilateral granular opacities and low lung volumes (Fig. 3-6), the identical findings in surfactant-deficient disease. The presence of pleural fluid is a helpful differentiating factor because it is very uncommon in surfactant deficiency but has been reported in between 25% and 67% of cases of β-hemolytic streptococcal pneumonia.
Persistent Pulmonary Hypertension in the Neonate
Persistent pulmonary hypertension in the neonate, also referred to as persistent fetal circulation, is a term often used in the NICU and is addressed here because it can be a source of confusion. The high pulmonary vascular resistance that is normally present in the fetus typically decreases during the newborn period. When this fails to happen, the pulmonary pressures remain abnormally high, and the condition is referred to as persistent pulmonary hypertension. It is a physiologic finding rather than a specific disease. It can be a primary phenomenon or it can occur secondary to causes of hypoxia, such as meconium aspiration syndrome, neonatal pneumonia, or pulmonary hypoplasia associated with congenital diaphragmatic hernia. These patients are quite ill. The radiographic patterns are variable and are more often reflective of the underlying cause of hypoxia than the presence of persistent pulmonary hypertension.
Neonatal Intensive Care Unit Support Apparatus
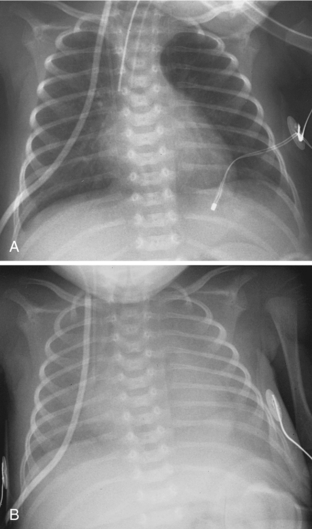
One of the primary roles of chest radiography in the NICU is to monitor support apparatus. They include endotracheal tubes, enteric tubes, central venous lines, umbilical arterial and venous catheters, and extracorporeal membrane oxygenation (ECMO) catheters. The radiographic evaluation of many of these tubes is the same as that seen in adults and is not discussed here. When evaluating the positions of endotracheal tubes in premature neonates, it is important to consider that the length of the entire trachea may be only about 1 cm. Keeping the endotracheal tube in the exact center of such a small trachea is an impossible task for caregivers, and phone calls and reports suggesting that the tube needs to be moved 2 mm proximally may be more annoying than helpful. Direct phone communication may be more appropriately reserved for times when the tube is in a main bronchus or above the thoracic inlet. There is an increased propensity to use esophageal intubation in neonates compared to its use in adults. Although it would seem that esophageal intubation would be incredibly obvious clinically, this is not always the case. I have seen cases in which a child has in retrospect been discovered to have been esophageally intubated for more than 24 hours. Therefore, the radiologist may be the first to recognize esophageal intubation. Obviously, when the course of the endotracheal tube does not overlie the path of the trachea, the use of esophageal intubation is fairly obvious. Other findings of esophageal intubation include a combination of low lung volumes, gas within the esophagus, and gaseous distention of the bowel (Fig. 3-7).
Umbilical Arterial and Venous Catheters
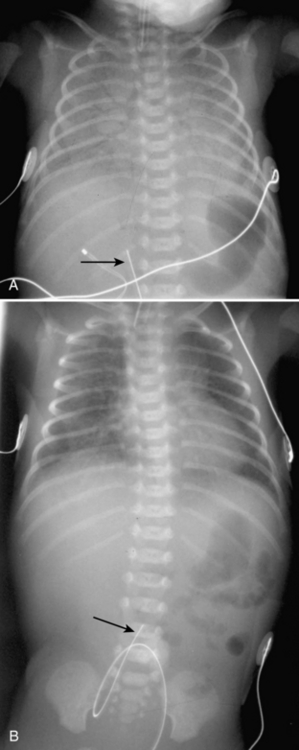
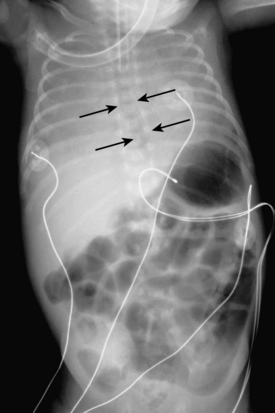
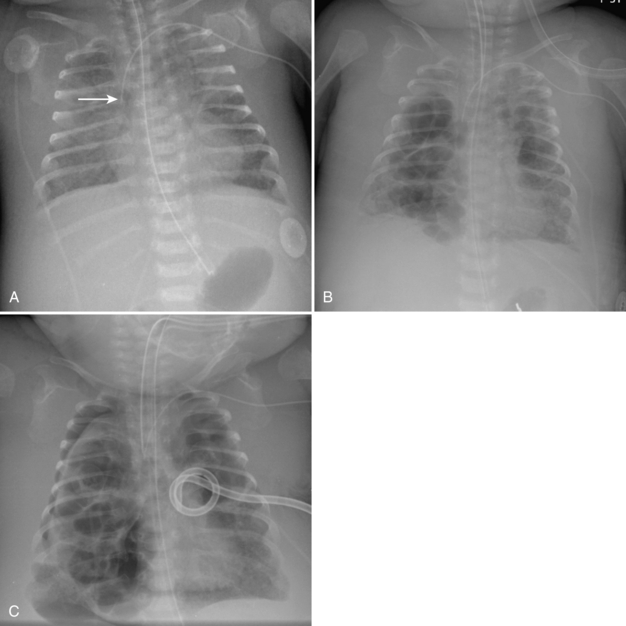
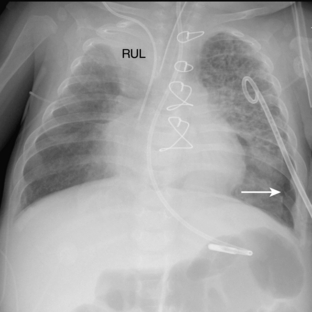
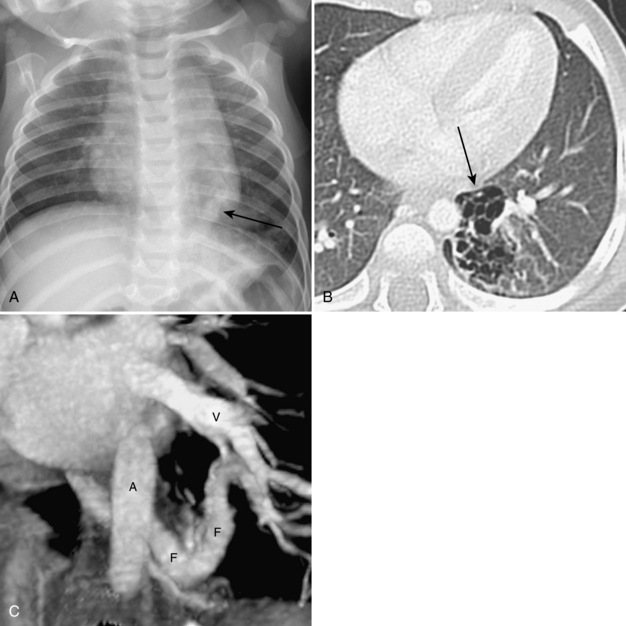
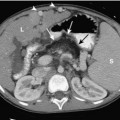
Umbilical arterial and venous catheters are commonly used in the NICU. Umbilical arterial catheters pass from the umbilicus inferiorly into the pelvis via the umbilical artery to the iliac artery. The catheters then turn cephalad within the aorta (see Fig. 3-5). These catheters can be associated with thrombosis of the aorta and its branches. Therefore, it is important to avoid positioning the catheter with the tip at the level of the branches of the aorta (celiac, superior mesenteric, and renal arteries). There are two acceptable umbilical arterial catheter positions: high lines have their tips at the level of the descending thoracic aorta (T8-T10; see Fig. 3-9); low lines have their tips below the level of L3 (see Fig. 3-5). The catheter tip should not be positioned between T10 and L3 because of the risk for major arterial thrombosis. There is no clear consensus as to whether a high or a low umbilical artery catheter line is better, and both positions are still currently used.
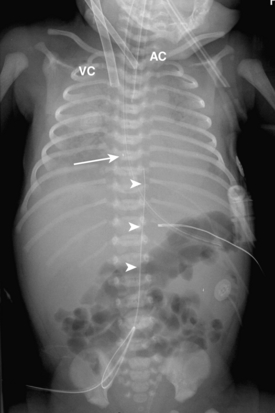
FIGURE 3-9. ECMO catheter placement for meconium aspiration syndrome (same child as in Fig. 3-1). Note venous ECMO catheter (VC) has a radiopaque proximal portion and a lucent distal portion. The tip of the venous catheter is marked by a small radiopaque metallic marker (arrow) and is actually in the right atrium. Note the arterial ECMO catheter (AC) with tip in region of aortic arch. Also, note “high”-type umbilical arterial catheter with tip overlying descending aorta at the level of T8.
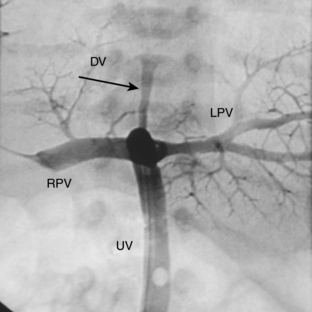
The pathway of the umbilical venous catheter is umbilical vein to left portal vein to ductus venosus to hepatic vein to inferior vena cava (Fig. 3-8). In contrast to umbilical arterial catheters, the course is in the superior direction from the level of the umbilicus. The ideal position of an umbilical venous catheter is with its tip at the junction of the right atrium and the inferior vena cava at the level of the hemidiaphragm (see Figs. 3-4, 3-5). The umbilical venous catheter may occasionally deflect into the portal venous system rather than passing into the ductus venosus. Complications of such positioning can include hepatic hematoma or abscess.
Peripherally Inserted Central Catheters in Children
One of the more common lines now seen in children, as in adults, is peripherally inserted central catheters (PICCs). In contrast to adults, in whom some of the PICCs can be as large as 6F, the PICC lines used in children, particularly infants, are often small in caliber (2F or 3F) so that they can be placed into their very small peripheral veins. These small caliber PICCs can be very difficult to see on chest radiography, so some of them must be filled with contrast to be accurately visualized. The tip of the PICC line that enters the child from the upper extremity or scalp should be positioned with the tip in the midlevel of the superior vena cava (see Fig. 3-27). It is essential that PICC lines not be left in place with the tip well into the right atrium. Particularly with the small-caliber lines, the atrium can be lacerated, leading to pericardial tamponade, free hemorrhage, or death. Many such cases have been reported nationally. Also, the PICC should not be too proximal in the superior vena cava because the distal portion of the line can flip from the superior vena cava into the contralateral brachiocephalic or jugular vein. At Cincinnati Children’s Hospital Medical Center, the PICC lines are inserted in a dedicated interventional radiology suite by a team of nurses, with supervision by pediatric interventional radiologists. Ultrasound is often used to guide vein cannulation and certified Child Life Specialists coach most kids through the procedure without having to sedate them. Fluoroscopy is utilized at the end of the procedure to adjust and document tip position in the mid-superior vena cava.
EXTRACORPOREAL MEMBRANE OXYGENATION
There are two types of ECMO: arteriovenous and venovenous. In ateriovenous ECMO, the right common carotid artery and internal jugular veins are sacrificed. The arterial catheter is placed via the carotid and positioned with its tip overlying the aortic arch. The venous catheter is positioned with its tip over the right atrium (Fig. 3-9). One of the main roles of a chest radiograph of children on ECMO is to detect any potential migration of the catheters. Careful comparison with previous studies to make sure that the catheters are not coming out or moving too far in is critical. These patients have many bandages and other items covering the external portions of the catheters, so migration may be hard to detect on physical examination. Note that there are various radiographic appearances of the ECMO catheters. Some catheters end where the radiopaque portion of the tube ends, and others have a radiolucent portion with a small metallic marker at the tip (see Fig. 3-9). It is common to see white-out of the lungs soon after a patient is placed on ECMO as a result of decreased ventilator settings and third-space shifting of fluid (see Fig. 3-9). Patients on ECMO are anticoagulated and are therefore at risk for hemorrhage.
Pulmonary Interstitial Emphysema
In patients with severe surfactant deficiency, ventilatory support can result in marked increases in alveolar pressure, leading to perforation of alveoli. The air that escapes into the adjacent interstitium and lymphatics is referred to as pulmonary interstitial emphysema (PIE). PIE appears on radiographs as bubblelike or linear lucencies and can be focal or diffuse (Fig. 3-10). The involved lung is usually noncompliant and is seen to have a static volume on multiple consecutive films. The finding is typically transient. The importance of detecting PIE is that it serves as a warning sign for other impending air-block complications such as pneumothorax, and its presence can influence caregivers in decisions such as switching from conventional to high-frequency ventilation.
It can be difficult to differentiate diffuse PIE from the bubblelike lucencies that are associated with developing bronchopulmonary dysplasia. When encountering this scenario, the patient’s age can help to determine which is more likely. Most cases of PIE occur in the first week of life, a time at which bronchopulmonary dysplasia is very unlikely. In patients older than 2 weeks, bronchopulmonary dysplasia is more likely. Also, in patients who have undergone a series of daily films, PIE may be noted to occur abruptly, whereas bronchopulmonary dysplasia tends to occur gradually. As previously mentioned, SDD partially treated by surfactant replacement can cause a pattern of lucencies that may mimic PIE as well.
Causes of Acute Diffuse Pulmonary Consolidation
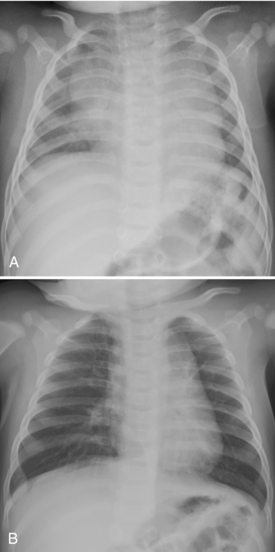
Acute diffuse pulmonary consolidation is nonspecific in neonates, as it is in adults, and can represent blood, pus, or water. In the neonate, the specific considerations include edema, which may be secondary to the development of patent ductus arteriosus (Fig. 3-11); pulmonary hemorrhage, to which surfactant therapy predisposes; worsening surfactant deficiency (during the first several days of life but not later); or developing neonatal pneumonia (Table 3-2). Diffuse microatelectasis is another possibility because neonates have the propensity to artifactually demonstrate diffuse lung opacity on low lung volume films (expiratory technique; Fig. 3-12A. B); this should not be mistaken for another cause of consolidation. Such radiographs showing low lung volumes offer little information concerning the pulmonary status of the patient and should be repeated when clinically indicated.
TABLE 3-2. Causes of Acute Diffuse Pulmonary Consolidation in Neonates
| Edema: patent ductus arteriosus |
| Hemorrhage |
| Diffuse microatelectasis: artifact |
| Worsening surfactant deficiency (only during first days of life) |
| Pneumonia |
Bronchopulmonary Dysplasia
BPD is related to injury to the lungs that is thought to result from some combination of mechanical ventilation and oxygen toxicity. Although four discrete and orderly stages of the development of BPD were originally described, they are not seen commonly and are probably not important to know. BPD typically occurs in a premature infant who requires prolonged ventilator support. At approximately the end of the second week of life, persistent hazy density appears throughout the lungs. Over the next weeks to months, a combination of coarse lung markings, bubblelike lucencies, and asymmetric aeration can develop (Fig. 3-13). Eventually, focal lucencies, coarse reticular densities, and bandlike opacities develop. In childhood survivors of BPD, many of these radiographic findings decrease in prominence over the years and only hyperaeration may persist. The radiographic findings may completely resolve. Clinically, many children with severe BPD during infancy may eventually improve to normal pulmonary function or may only have minor persistent problems such as exercise intolerance, predisposition to infection, or asthma.
Focal Pulmonary Lesions in the Newborn
In contrast to diffuse pulmonary disease in newborns, focal masses can present with respiratory distress due to compression of otherwise normal lung. Most of these focal masses are related to congenital lung lesions. Congenital lung lesions may appear solid, as air-filled cysts, or mixed in appearance. The differential for a focal lung lesion can be separated on the basis of whether the lesion is lucent or solid appearing on chest radiography (Table 3-3). The most likely considerations for a lucent chest lesion in a newborn are congenital lobar emphysema, congenital cystic adenomatoid malformation, persistent pulmonary interstitial emphysema, and congenital diaphragmatic hernia. CT may be helpful in differentiating among these lesions by demonstrating whether the abnormal lucency is related to air in distended alveoli, in the interstitium, or in abnormal cystic structures. Lesions that typically appear solid during the neonatal period include sequestration and bronchogenic cyst. Many of these lesions can present in children beyond the neonatal period, and those aspects of these entities are also discussed here.
TABLE 3-3. Focal Lung Lesions in Neonates on Radiography
| Lucent Lesions | Solid Lesions |
|---|---|
The following sections are divided into specific congenital lesions. However, it has been increasingly recognized that there can be “mixed” lesions, which show characteristics of more than one type of lesions (see Fig. 3-16). The most common mixed lesions are those that show characteristics of both congenital cystic adenomatoid malformation and sequestration.