CHAPTER EIGHT Neuro
Pediatric neuroimaging is a distinct subspecialty. Anatomic areas included in neuroimaging include the skull, brain, meninges, orbits, sinuses, neck, and spine. At many children’s hospitals, dedicated neuroradiologists perform and interpret all of the neuroimaging. The large amount of information included in neuroradiology is beyond the scope of this textbook. However, this chapter is a review of some of the more commonly encountered entities.
BASIC REVIEW OF ADVANCED MRI TECHNIQUES IN PEDIATRIC NEUROIMAGING
Functional MRI
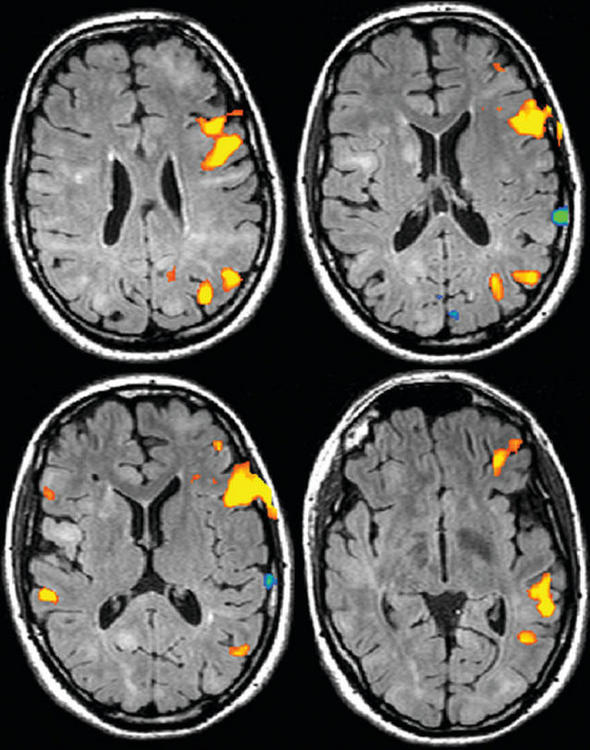
Functional MRI (fMRI) is a noninvasive method of evaluating regional neuronal activity within the brain. Neuronal activity increases metabolic activity, which results in increased blood flow to that region and a relative increase in the ratio of oxygenated hemoglobin to deoxygenated hemoglobin. Deoxyhemoglobin is paramagnetic; therefore, a change in the ratio affects the magnetic state of the tissue and as a result, changes the local MRI signal. This phenomenon is called the BOLD (blood oxygenation level-dependent) effect. fMRI techniques show these changes superimposed on anatomic information. fMRI is used to document regional neuronal activity during a specific activity: language (Fig. 8-1), memory, or motor function. It has been a very useful research tool in increasing our understanding of brain function during various tasks. Clinically, fMRI has been used in areas such as the evaluation of patients with seizures and the planning of surgical approaches to brain tumors.
MR Spectroscopy
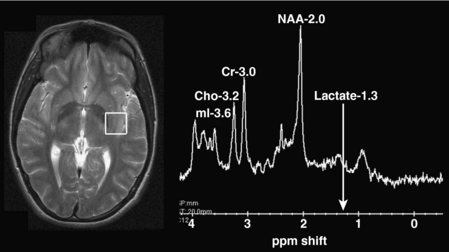
Proton (hydrogen) MR spectroscopy is a tool that is now routinely used to help characterize a number of pediatric neurologic conditions. Most often, single-voxel spectroscopy is created using a 1 cubic centimeter sample area. The results of spectroscopy are typically depicted as a spectrum, with each metabolic peak characterized by resonance frequency, height, width, and area (Fig. 8-2). Metabolic profiles depicted on MR spectroscopy have been much less specific than originally hoped. However, the information obtained does provide useful information that complements the information derived from MRI in many pediatric disorders, including neoplasms, ischemia, and white matter diseases.
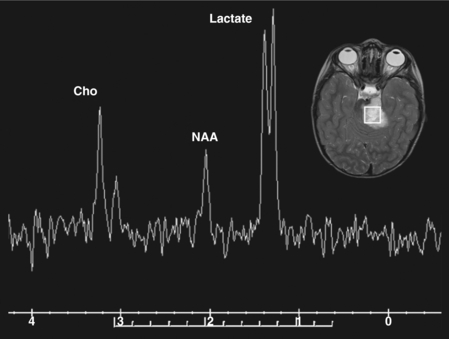
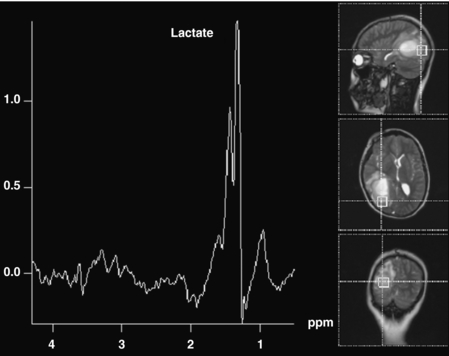
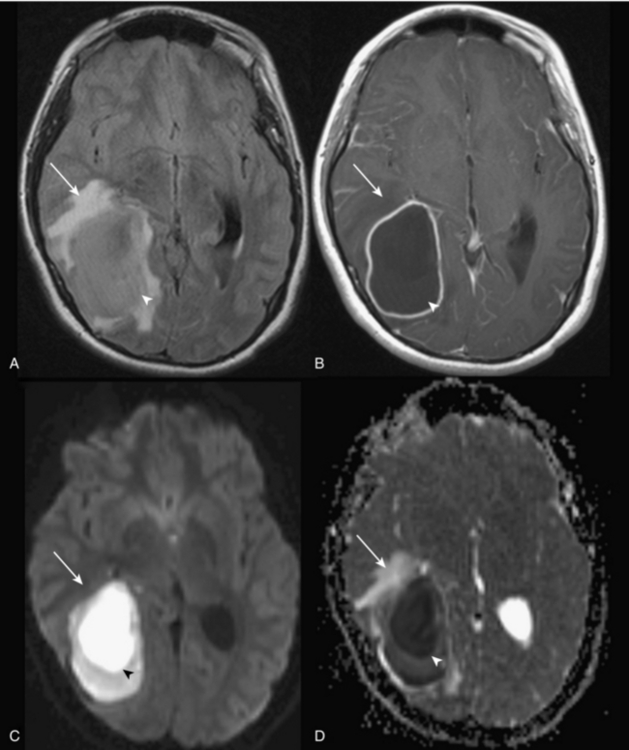
The commonly evaluated brain metabolites include N-acetyl aspartate (NAA), creatine, choline, and lactate (see Fig. 8-2). NAA is a neuronal marker and is decreased in most disorders that destroy brain tissues, such as neoplasms (Fig. 8-3), infarcts, radiation injuries, and seizures. NAA is markedly elevated in Canavan disease. Choline compounds reflect the synthesis and degradation of cell membranes; therefore, increased choline is seen when there is high cellular turnover, as occurs with most tumors (see Fig. 8-3). Lactate is typically elevated in the setting of acute ischemia, infarction, and many high-grade tumors and severe infections (Fig. 8-4; and see Fig. 8-3). Creatine remains stable in the presence of many disorders but can be increased in hypometabolic states and decreased in hypermetabolic states.
Diffusion-Weighted Imaging
Diffusion-weighted imaging (DWI) images are determined by the variability in the diffusivity of water in tissues. Molecules move randomly in fluid (Brownian motion). In tissues, there is variable restriction of the movement of water molecules relative to tissue structure (cell membranes, vascular structures, density of cells, axons). Pathologic states alter the diffusion characteristics of brain water and therefore affect the appearance of the image. DWI images are created by applying two additional magnetic field gradients. The first dephases the spins and the second rephases the spins, if no movement occurs. If there is free movement of molecules, such as in the free-moving water in the cerebrospinal fluid (CSF), the protons lose spin coherence and there is signal loss. If movement is restricted, the movement of molecules is less random and more signal is returned.
Images in DWI are influenced not only by diffusion but also by other tissue properties such as T2. In order to eliminate the influences on imaging appearance of factors unrelated to diffusion, the data are frequently processed, most commonly by a technique called an apparent diffusion coefficient (ADC) map. Therefore, for each DWI sequence, two sets of images are often created: the DWI images and the ADC map. What the bright and low areas mean on these two sets of images can be confusing, because they are the opposites of each other. In a pathologic process in which there is restricted diffusion, the involved area appears high in signal on the DWI images and low in signal on the ADC map. Therefore, for clarification: restricted diffusion = bright signal on DWI = dark signal on ADC map = diminished ADC. Conversely, in pathologic processes in which there is increased diffusion, there is a dark signal on DWI images and a high signal on the ADC map. Table 8-1 shows a glossary of terms related to DWI and diffusion tensor imaging (DTI).
Table 8-1. Glossary of Terms in Diffusion-Weighted and Diffusion Tensor Imaging
| Term | Definition |
|---|---|
| Isotropy | Uniformity of physical properties of a molecule in all directions; in other words, the absence of any kind of polarity |
| Anisotropy | The opposite of isotropy; having polarity or directionality |
| Eigenvalue | The mathematical property of a tensor (vector) related to magnitude and direction |
| Fractional anisotropy | A metric measuring the degree of anisotropy (0 for isotropy and 1 for full anisotropy) |
| Apparent diffusion coefficient (ADC) | A measure of the freedom of water diffusion in a particular tissue; increased ADC = increased diffusivity |
| Tractography | A postprocessing method of creating images representing axonal fiber tracts from diffusion tensor data |
| Tensor | The magnitude and direction of diffusion; is used similarly to the term vector. A 3 × 3 matrix is used to calculate a tensor |
Pathologic processes that show restricted diffusion include acute infarction; cellular edema (such as that caused by acute ischemia or nonhemorrhagic traumatic injury); purulent fluid collections (Fig. 8-5); epidermoid cysts; and hypercellular tumors such as medulloblastoma. Processes that show increased diffusion include vasogenic edema and CSF collections such as those in an arachnoid cyst.
Diffusion Tensor Imaging
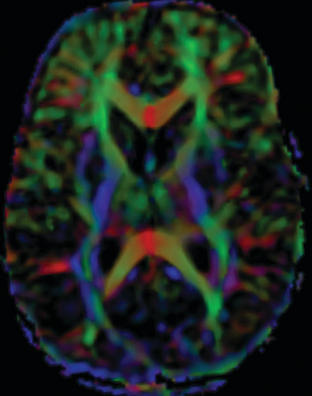
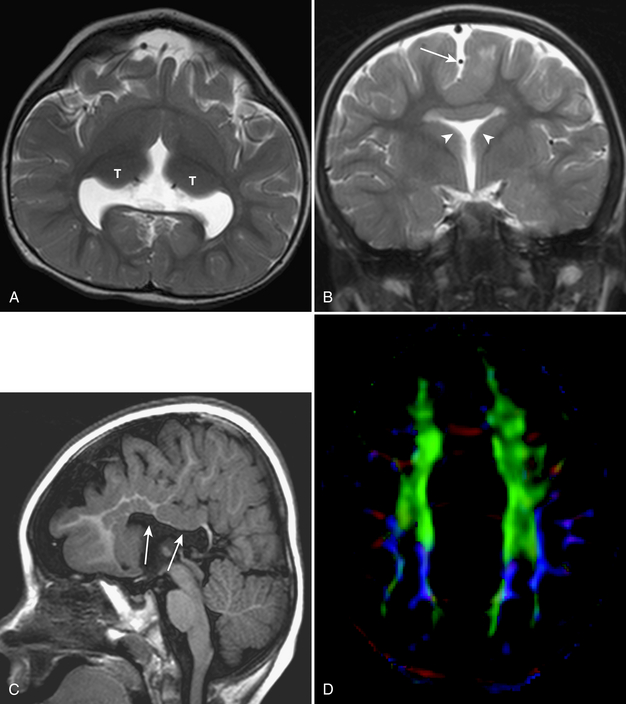
Tractography images are typically shown in color. By convention, white matter tracts oriented left to right are shown as red, cephalocaudad as blue, and anterioposterior as green (Fig. 8-6).
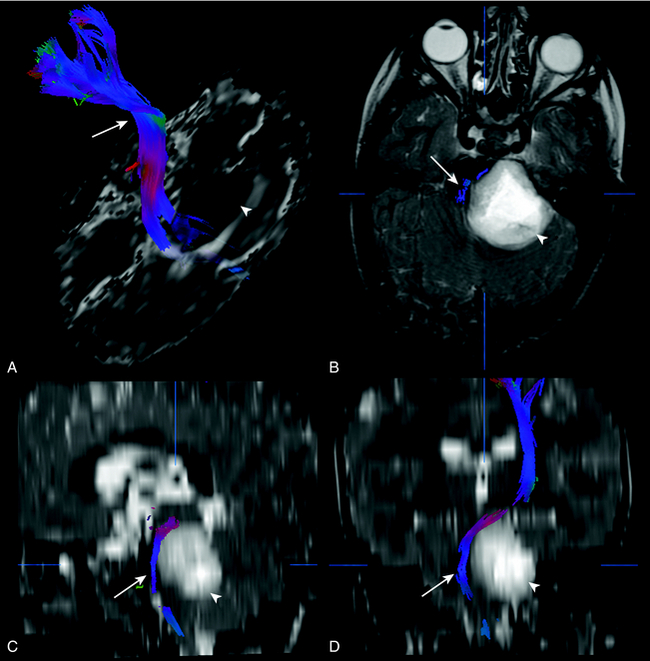
DTI and tractography can be used to evaluate myelination. Increased myelination increases anisotrophy. Therefore, in normal infants, anisotrophy increases with age. Most disease states that affect white matter decrease anisotrophy. DTI has been used to evaluate stroke, brain tumor (Fig. 8-7), trauma, and demyelinating disorders. DTI and tractography have also been used to study and depict the white matter tract abnormalities associated with congenital brain anomalies and in the presurgical evaluation of brain tumors (see Fig. 8-7).
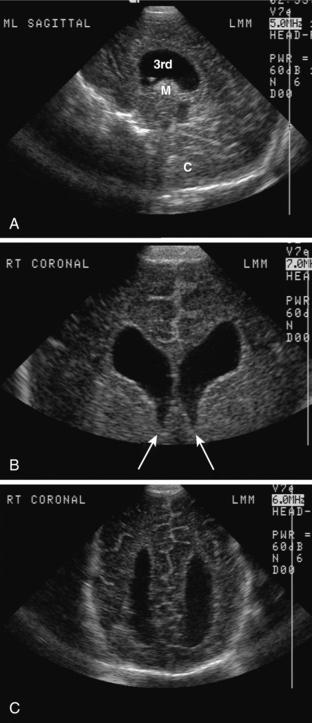
NEONATAL HEAD ULTRASOUND
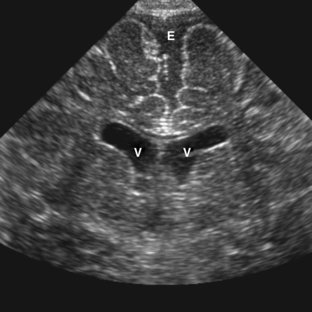
Neonatal head ultrasound is performed through the open anterior fontanel of neonates and infants using a high-frequency sector transducer. Images are commonly obtained in the sagittal and coronal planes (Fig. 8-8). Head ultrasound is most commonly used to diagnose and follow up, in premature infants, intracranial complications, such as germinal matrix hemorrhage and periventricular leukomalacia. It can also be used to screen for congenital abnormalities and hydrocephalus. Another common indication is evaluation of an infant with a large head circumference.
Germinal Matrix Hemorrhage
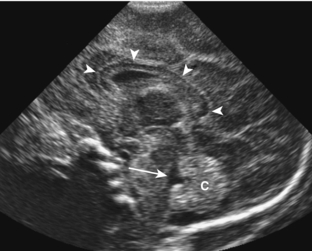
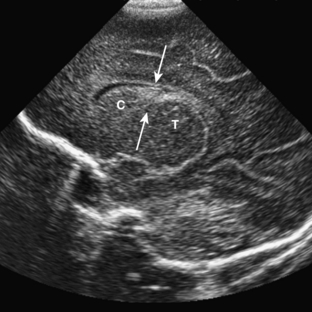
On ultrasound, germinal matrix hemorrhage is seen as an ovoid echogenic mass within the caudothalamic groove (Figs. 8-9, 8-10A, B, 8-11). For those not well acquainted with head ultrasound, there may be confusion in differentiating germinal matrix hemorrhage from the normally echogenic choroid plexus. In contrast to germinal matrix hemorrhage, normal choroid plexus should never extend as anterior as the caudothalamic groove on a parasagittal view. Hemorrhage may extend into the ventricular system and lead to hydrocephalus. Germinal matrix hemorrhage is categorized into one of four grades (Table 8-2). Intraparenchymal hemorrhage (grade IV) is actually thought to be secondary to venous infarction rather than a direct extension of hemorrhage (see Fig. 8-11). Grades I and II hemorrhages tend to have good prognoses. In contrast, grades III and IV hemorrhages tend to have poor prognoses, including high incidences of neurologic impairment, hydrocephalus, and death.
Table 8-2. Grades of Germinal Matrix Hemorrhage
| Grade | Morphologic Findings |
|---|---|
| I | Hemorrhage confined to germinal matrix |
| II | Intraventricular hemorrhage without ventricular dilatation |
| III | Intraventricular hemorrhage with ventricular dilatation |
| IV | Intraparenchymal hemorrhage |
Periventricular Leukomalacia
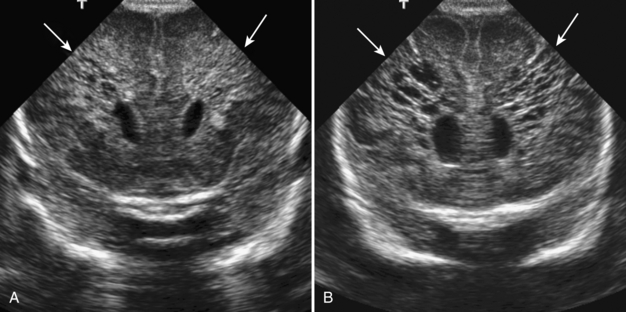
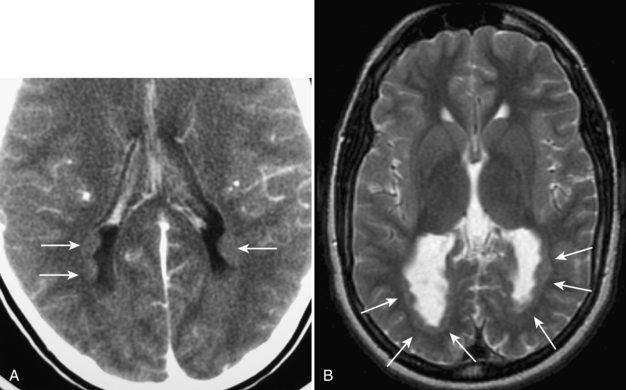
Perinatal partial asphyxia can result in damage to the periventricular white matter, the watershed zone of the premature infant. This is termed periventricular leukomalacia. It most commonly affects the white matter adjacent to the atria and the frontal horns of the lateral ventricles. It is associated with neurologic sequelae, such as movement disorders, seizures, and spasticity. On ultrasound, increased heterogeneous echogenicity is seen within the periventricular white matter. In severe cases, there may be cystic necrosis and development of periventricular cysts (Fig. 8-12A, B). With time, there is often volume loss of the involved white matter (Fig. 8-13).
Benign Macrocrania
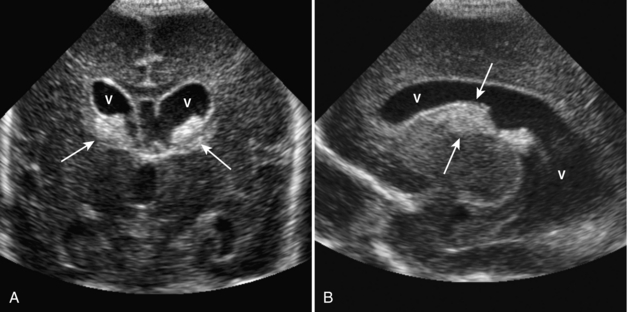
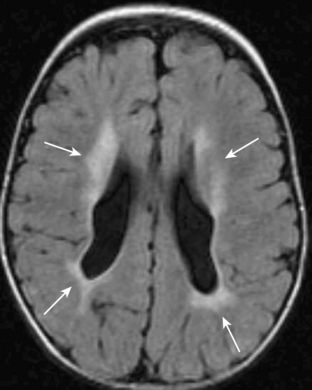
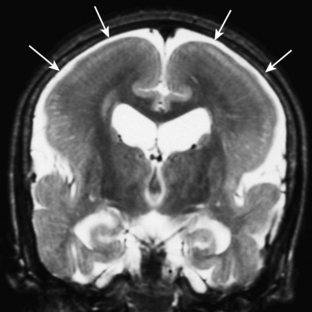
Benign macrocrania is a diagnosis of exclusion. The term refers to children with large heads (head circumference greater than 97% for age). Typically, such children present between 6 months and 2 years of age. After 2 years of age, the head size usually normalizes and the children have no long-term consequences. The parents of such children often have large heads or had large heads as infants. On imaging, there is prominent size of the lateral ventricles and extraaxial spaces (Fig. 8-14). Imaging is otherwise normal. Imaging findings alone cannot differentiate between benign macrocrania and communicating hydrocephalus.
DEVELOPMENTAL ABNORMALITIES
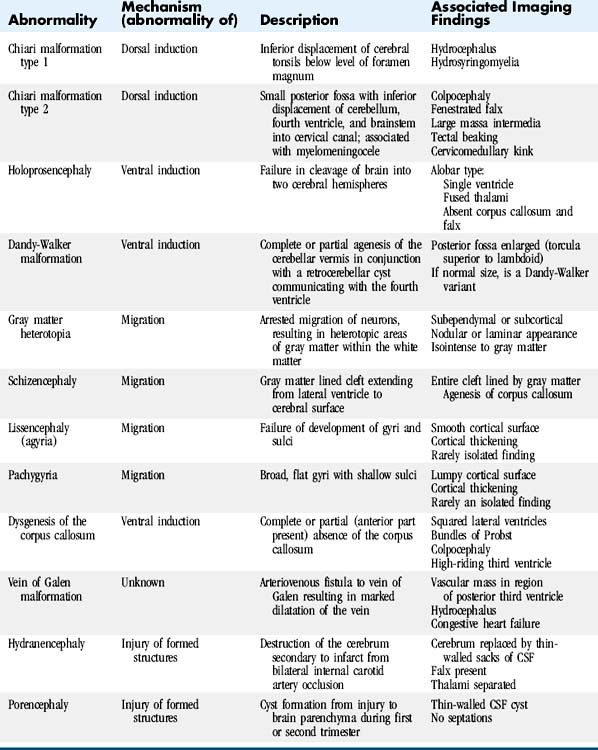
Developmental abnormalities can be classified on the basis of the embryologic event that fails, causing the abnormality (Table 8-3). Categories include abnormalities of dorsal induction, ventral induction, migration and cortical organization, neuronal proliferation and differentiation, and myelination. Abnormalities can also result from destruction of already formed structures. The type of developmental lesion often reflects the timing of the disturbance that occurred during development. Often, multiple distinct developmental abnormalities are present simultaneously.
Chiari Malformations
CHIARI MALFORMATION TYPE 1
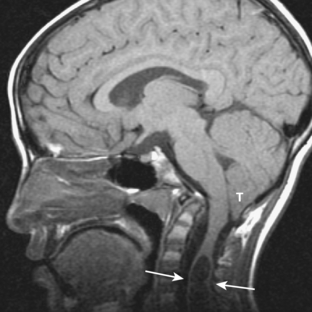
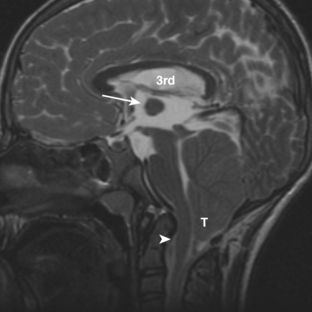
Chiari malformation type 1 is the presence of an abnormally inferior location of the cerebellar tonsils at least 5 mm below the foramen magnum (Fig. 8-15). The medulla and fourth ventricle are in normal positions. Complications of Chiari type 1 malformation include hydrocephalus and hydrosyringomyelia (up to 25%; see Fig. 8-15). It may be suspected on CT when the foramen magnum appears “full” of soft tissue. It is best visualized on sagittal T1-weighted MR images. The cerebellar tonsils are seen positioned inferiorly and typically appear elongated rather than round. MR cine sequences are used to show movement of the tonsils into the foramen magnum.
CHIARI MALFORMATION TYPE 2
Chiari malformations type 2 are almost always associated with myelomeningoceles. Conversely, almost all patients with myelomeningoceles have Chiari type 2 malformations. Patients with these lesions have small posterior fossae and associated inferior displacement of the cerebellum, medulla, and fourth ventricle into the upper cervical canal (Figs. 8-16, 8-17). Associated imaging findings include a kinked appearance of the medulla, colpocephaly (disproportionate enlargement of the posterior body of the lateral ventricles), fenestration of the falx associated with interdigitation of gyri across the midline, enlargement of the massa intermedia, inferior pointing of the lateral ventricles, and tectal beaking (a pointed appearance of the quadrigeminal plate). Chiari type 2 malformations are usually associated with hydrocephalus.
Holoprosencephaly
Holoprosencephaly results from lack of cleavage of the brain into two hemispheres. Although there is a continuous spectrum of severity, holoprosencephaly is classically classified into one of three distinct groups: alobar, semilobar, or lobar. The severity of the brain abnormality is reflected in the severity of the midline facial abnormality.
With the intermediate form, semilobar holoprosencephaly (Fig. 8-18A-D), the cerebral hemispheres are partially cleaved from each other posteriorly. The temporal horns may be formed but there is a single ventricle anteriorly. There is partial separation of the thalami. Midline structures such as the falx and corpus callosum may be present posteriorly but not anteriorly.
Lobar holoprosencephaly is the least severe form. The occipital and temporal horns are well formed but there is failure of cleavage of the frontal lobes. The septum pellucidum is absent and the corpus callosum may be absent or dysplastic.
Posterior Fossa Cystic Malformations
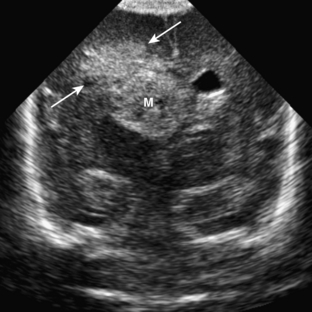
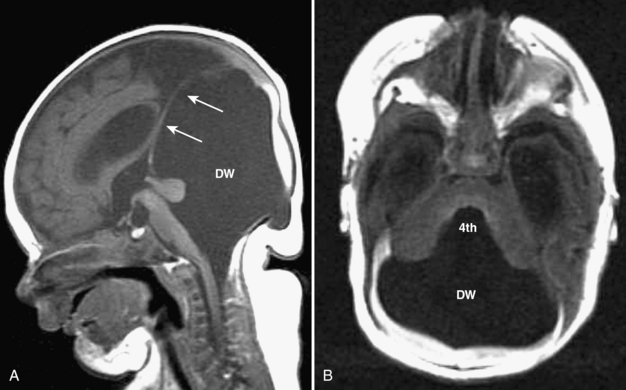
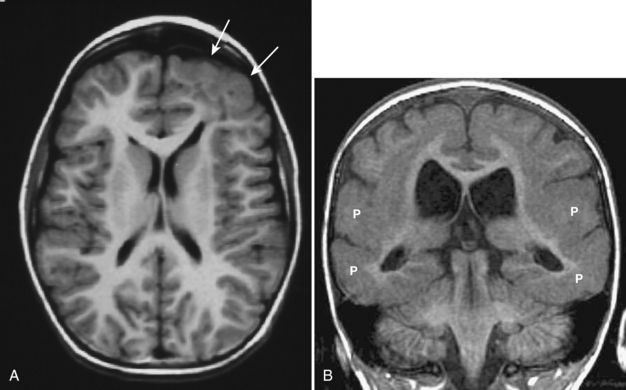
Dandy-Walker malformation is complete or partial agenesis of the cerebellar vermis in conjunction with the presence of a posterior fossa cyst that communicates with the fourth ventricle (Fig. 8-19A, B). The posterior fossa is enlarged such that the torcular is elevated above the lambda (see Fig. 8-19). The falx cerebelli is absent. Often there are also supratentorial abnormalities, including holoprosencephaly, agenesis of the corpus callosum, polymicrogyria, and heterotopias. Hydrocephalus is common.
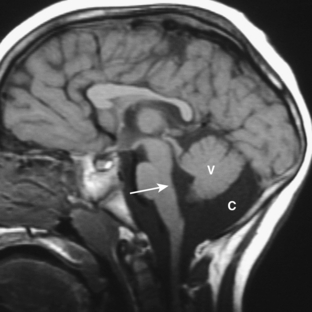
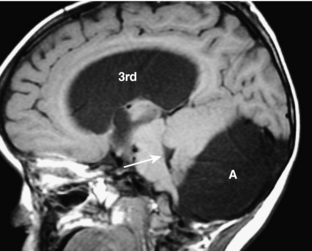
Dandy-Walker variant is considered present when some but not all of the criteria for the classic malformation are present. Most commonly, the cerebellar vermis is hypoplastic but present, and there is a posterior fossa cyst, but the posterior fossa is not enlarged (Fig. 8-20). When an enlarged posterior fossa CSF cyst is present in the presence of a fully developed cerebellar vermis, there are two possibilities. If the cyst exhibits no mass effect on the cerebellum, a mega cisterna magna is considered to be present. If the cyst does exhibit mass effect on the cerebellum, an arachnoid cyst is considered to be present (Fig. 8-21).
Gray Matter Heterotopias
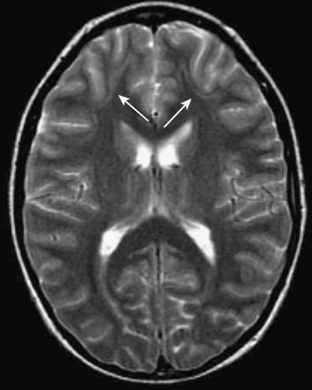
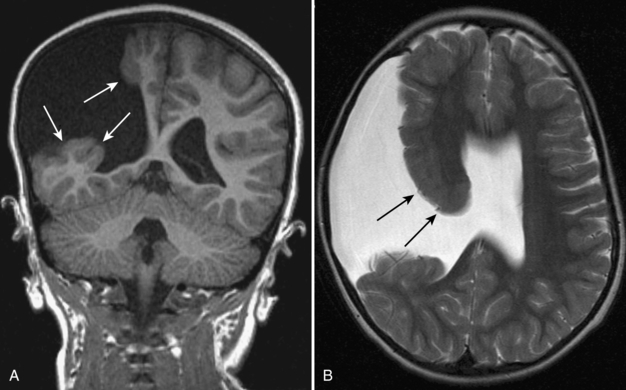
Heterotopias are abnormalities of neuronal migration characterized by arrest in migration of the neurons from the subependymal area to the cortex. Typically, heterotopias are associated with other migrational disorders, such as schizencephaly, lissencephaly, or polymicrogyria. When a heterotopia occurs as an isolated abnormality, it typically presents with focal seizures. On CT and MRI, the lesions appear as nodular (Fig. 8-22A, B) or linear (Fig. 8-23) areas within the white matter, most typically in the subcortical or subependymal regions. Heterotopias tend to be isointense, with gray matter on all pulse sequences.
Schizencephaly
Schizencephaly is another migrational disorder. The term refers to a cleft in the cerebral hemisphere lined with gray matter (Fig. 8-24A, B). The cleft typically extends from the lateral ventricle to the surface of the brain. Schizencephaly is often characterized as being open-lipped or close-lipped. However, this has little clinical relevance. The lesion can be unilateral or bilateral and can occur anywhere in the cerebral hemispheres. In most cases, there is associated agenesis of the corpus callosum.
Lissencephaly
Lissencephaly refers to arrest of migration of neurons, resulting in either total failure of development of sulci and gyri (agyria; Fig. 8-25) or development of abnormally broad and flat gyri with abnormally shallow sulci (pachygyria) (Fig. 8-26A, B). Agyria and pachygyria are best visualized with MR imaging. There is often thickening of the associated cortex. Neither lesion typically appears in isolation; there are usually patchy areas of both agyria and pachygyria. These abnormalities are commonly associated with other migrational abnormalities and also occur as part of a number of rare syndromes.
Dysgenesis of Corpus Callosum
Dysgenesis of the corpus callosum includes both complete and partial absence. The corpus callosum normally develops from anterior to posterior. As a result, with partial absence, it is the more anterior part of the corpus callosum that is present. Absence of the corpus callosum can occur as an isolated lesion or in conjunction with many of the other developmental lesions of the brain already described in this chapter (see Fig. 8-18). On coronal MR images, the lateral ventricles are separated, and the third ventricle extends more superiorly than normal, positioned between the lateral ventricles. The white matter tracts that normally cross the midline via the corpus callosum run along the medial surface of the lateral ventricles and form the bundles of Probst, which can be seen at imaging. Colpocephaly is often present. Midline masses, such as lipoma and arachnoid cyst, can also be associated.