Dieulafoy disease = abnormally dilated submucosal vessels of pulmonary artery prone to hemorrhage
[Paul Georges Dieulafoy (1839–1911), chief of medical services at the Hôtel-Dieu, Paris, France]
2. Bronchiectasis, mouthful (15%)
3. Tuberculosis (Rasmussen aneurysm)
4. Aspergillosis
5. Abscess
6. Cystic fibrosis
E. CRYPTOGENIC (3–10–42%)
◊ 6–10% of patients who smoke present with unresectable lung cancer within subsequent 3 years!
CT:
√ cluster of avidly enhancing “nodular” bronchial arteries in posterior mediastinum below level of aortic arch paralleling bronchial anatomy
√ abnormal bronchial arteries show tortuosity, dilatation, hypervascularity, neovascularity, aneurysms, shunts
N.B.: multidetector CT mapping prior to angiography reduces the rate of catheterization failures and the number of patients needing surgical intervention!
Angio:
√ vasodilated enlarged tortuous bronchial arteries → bronchial-to-pulmonary-artery shunting, hypervascularity, parenchymal staining
Prognosis: 50–100% mortality rate of conservatively treated massive hemoptysis; death usually from asphyxiation rather than from exsanguination
Rx: transcatheter particulate embolization of bronchial aa. using polyvinyl alcohol (PVA) + Gelfoam® pledgets (effective in 70–95%; recurrent bleeding in 20–30%)
N.B.: identify arteria radicularis (= artery of Adamkiewicz) at T5–L2 (in 75% T9–T12) to avoid postembolization transverse myelitis
DDx: hematemesis (containing food particles, dark blood, acid pH)
ASPIRATION
= intake of solid / liquid materials into the airways and lungs
Predisposing factors:
1. Alcoholism (most common in adults)
2. General anesthesia, loss of consciousness
3. Structural abnormalities of pharynx / esophagus (congenital / acquired tracheoesophageal + tracheopulmonary fistula), laryngectomy
4. Neuromuscular disorders
5. Deglutition abnormalities
Substrate:
(a) solids
› foreign bodies
› lentils
(b) liquids
› gastric acid = Mendelson syndrome
› water = near drowning
› barium, water-soluble contrast material
› liquid paraffin / petroleum = acute exogenous lipoid pneumonia / fire-eater pneumonia
› mineral oil / cod liver oil = chronic exogenous lipoid pneumonia
(c) contaminated substances from oropharynx / GI tract
Acute Lower Airway Obstruction in Childhood
Location: intrathoracic trachea + bronchi
(a) infectious / inflammatory:
• fever, cough, wheeze
› < 2 years old:
1. Bronchiolitis
√ bronchial wall thickening + hyperinflation
› > 2 years old:
2. Lower respiratory tract inflammation
√ bronchial wall thickening
3. Reactive airways disease
√ bronchial wall thickening + hyperinflation
(b) others:
1. Aspirated foreign body
PULMONARY DISEASE & CIGARETTE SMOKING
1. Bronchogenic carcinoma
2. Chronic bronchitis
3. Centrilobular emphysema
4. Panacinar emphysema with α-1 antitrypsin deficiency
5. Smoking-related interstitial lung disease
› Respiratory bronchiolitis ILD (RB-ILD)
› Desquamative interstitial pneumonitis (DIP)
› Pulmonary Langerhans cell histiocytosis (PLCH)
› Idiopathic pulmonary fibrosis (IPF)
HYPERSENSITIVITY TO ORGANIC DUSTS
A. TRACHEOBRONCHIAL HYPERSENSITIVITY
large particles reaching the tracheobronchial mucosa (pollens, certain fungi, some animal / insect epithelial emanations)
1. Extrinsic asthma
2. Hypersensitivity aspergillosis
3. Bronchocentric granulomatosis
4. Byssinosis in cotton-wool workers
B. ALVEOLAR HYPERSENSITIVITY
= HYPERSENSITIVITY PNEUMONITIS
= EXTRINSIC ALLERGIC ALVEOLITIS
small particles of < 5 µm reaching alveoli
DRUG-INDUCED PULMONARY DAMAGE
Histopathologic manifestations:
(a) Diffuse alveolar damage:
bleomycin, busulfan, carmustine, mitomycin, cyclophosphamide, melphalan, gold salts
(b) Nonspecific interstitial pneumonia:
amiodarone, methotrexate, carmustine, chlorambucil
(c) Bronchiolitis obliterans organizing pneumonia: gold salts, bleomycin, methotrexate, amiodarone, nitrofurantoin, penicillamine, sulfasalazine, cyclophosphamide
(d) Eosinophilic pneumonia:
penicillamine, sulfasalazine, nitrofurantoin, nonsteroidal anti-inflammatory drugs, para-aminosalicylic acid
(e) Pulmonary hemorrhage:
anticoagulants, amphotericin B, cytarabine (ara-C), penicillamine, cyclophosphamide
A. CYTOTOXIC DRUGS (most important group)
1. Cyclophosphamide
Use: variety of malignancies, Wegener granulomatosis, glomerulonephritis
Toxicity: after 2 weeks – 13 years (mean, 3.5 years), no relationship to dose / duration of therapy
Prognosis: good after discontinuation of therapy
√ diffuse alveolar damage (most common)
√ nonspecific interstitial pneumonia
√ BOOP (least common)
2. Busulfan = Myleran® (for CML)
Toxicity: dose-dependent, after 3–4 years on the drug in 1–10%
√ diffuse linear pattern (occasionally reticulonodular / nodular pattern)
√ partial / complete clearing after withdrawal of drug
DDx: Pneumocystis pneumonia, interstitial leukemic infiltrate
3. Nitrosoureas = carmustine (BCNU), lomustine (CCNU)
Use: CNS glioma, lymphoma, myeloma
Toxicity: in 50% after doses > 1500 mg/m2; sensitivity increased after radiation Rx
√ diffuse alveolar damage (most common)
√ nonspecific interstitial pneumonia:
√ linear / finely nodular opacities (following treatment of 2–3 years)
√ high incidence of pneumothorax
4. Bleomycin
Use: squamous cell carcinoma of neck / cervix / vagina, Hodgkin lymphoma, testicular carcinoma
Toxicity: at doses > 300 mg (in 3–6%); increased risk with age + radiation therapy + high oxygen concentrations
Prognosis: death from respiratory failure within 3 months of onset of symptoms
√ diffuse alveolar damage (most common)
√ nonspecific interstitial pneumonia / BOOP:
√ subpleural linear / nodular opacities (5–30 mm) in lower lung zones occurring after 1–3 months following beginning of therapy
DDx: metastases
5. Taxoid derivatives = paclitaxel, docetaxel, gemcitabine, topotecan, vinorelbine
Use: breast cancer, lung cancer, ovarian cancer
√ interstitial pneumonitis
B. NONCYTOTOXIC DRUGS
1. Amiodarone
= triiodinated benzofuran
Use: refractory ventricular tachyarrhythmia
Toxicity: in 5–10%; risk increased with daily dose > 400 mg + in elderly
Prognosis: good after discontinuation of drug
• pulmonary insufficiency after 1–12 months in 14–18% on long-term therapy
√ nonspecific interstitial pneumonia (most common) + associated BOOP:
√ alveolar + interstitial infiltrates (chronic presentation)
√ focal homogeneous peripheral consolidation (acute presentation):
√ attenuation values of iodine ← incorporation of amiodarone into type II pneumocytes
√ pleural thickening (inflammation) adjacent to consolidation
√ associated high-attenuation of liver relative to spleen
2. Gold salts
Use: inflammatory arthritis
Toxicity: in 1% within 2–6 months
• mucocutaneous lesions (30%)
√ diffuse alveolar damage (common)
√ nonspecific interstitial pneumonia (common)
√ BOOP (less common)
3. Methotrexate, procarbazine
Use: lung cancer, breast cancer, head and neck epidermoid cancer, nonmetastatic osteosarcoma, advanced stage NHL, AML, recalcitrant psoriasis, severe rheumatoid arthritis, pemphigus
Toxicity: in 5–10%; not dose-related
Prognosis: usually self-limited despite continuation of therapy
• blood eosinophilia (common)
√ nonspecific interstitial pneumonia (most common)
√ BOOP (less frequent)
√ linear / reticulonodular process (time delay of 12 days to 5 years, usually early)
√ acinar filling pattern (later)
√ transient hilar adenopathy + pleural effusion (on occasion)
DDx: Pneumocystis pneumonia
4. Nitrofurantoin (Macrodantin®)
Use: urinary tract infection
Toxicity: rare
• positive for ANA + LE cells
(a) acute disorder within 2 weeks of administration:
• fever, dyspnea, cough
• peripheral eosinophilia (more common)
Prognosis: prompt resolution after withdrawal from drug
√ diffuse bilateral predominantly basal heterogeneous opacities
(b) chronic reaction with interstitial fibrosis (less common)
• insidious onset of dyspnea + cough
• may not be associated with peripheral eosinophilia
√ nonspecific interstitial pneumonia (common)
√ bilateral basilar interstitial opacities
C. OTHERS
1. Heroin, propoxyphene, methadone
Toxicity: overdose followed by pulmonary edema in 30–40%
√ bilateral widespread airspace consolidation
√ aspiration pneumonia in 50–75%
2. Salicylates
• asthma
√ pulmonary edema (with chronic ingestion)
3. Intravenous contrast material
√ pulmonary edema
LYMPHOPROLIFERATIVE MALIGNANCIES
= subgroup of hematologic malignancies comprising 4 different types:
1. Lymphoma (Non-Hodgkin Lymphoma)
> 30 subtypes of lymphoma
2. Hodgkin disease
3. Lymphocytic leukemias (acute or chronic)
4. Plasma cell myeloma (multiple myeloma)
PULMONARY DISEASE AND OTHER ORGAN MANIFESTATIONS
Disorders with Hepatic & Pulmonary Manifestations
1. Alpha-1-antitrypsin deficiency
2. Cystic fibrosis
3. Hereditary hemorrhagic telangiectasia
4. Autoimmune disease: primary biliary cirrhosis, rheumatoid arthritis, Hashimoto thyroiditis, Sjögren syndrome, scleroderma, sarcoidosis
5. Drugs with toxic effects on lung and liver: methotrexate, phenytoin, amiodarone
Pulmonary-Renal Syndromes
1. Wegener granulomatosis = granulomatosis with polyangiitis
2. Goodpasture syndrome
3. Systemic lupus erythematosus
4. Mixed connective tissue disease
5. Microscopic polyangiitis
PULMONARY EDEMA
= abnormal accumulation of fluid in the extravascular compartments of the lung
Pathophysiology (Starling equation):
transcapillary flow dependent on
(1) Hydrostatic pressure
(2) Oncotic (= colloid osmotic) pressure
(3) Capillary permeability (the endothelial cells are relatively impermeable to protein but remain permeable to water and solutes; the tight intercellular junctions of alveolar epithelium remain nearly impermeable to water and solutes)
Qfilt = Kfilt (HPiv – HPev) – t(OPiv – OPev)
Cause: disturbed equilibrium of net flow Fnet between fluid transudation / exudation Qfilt and lymphatic absorption Qlymph
Fnet = Qfilt – Qlymph
A. INCREASED HYDROSTATIC PRESSURE
(a) cardiogenic (most common)
= PULMONARY VENOUS HYPERTENSION
1. Heart disease: left ventricular failure, mitral valve disease, left atrial myxoma
2. Pulmonary venous disease: acute / chronic pulmonary embolism, primary venoocclusive disease, mediastinal fibrosis
3. Pericardial disease: pericardial effusion, constrictive pericarditis (extremely rare)
4. Drugs: antiarrhythmic drugs; drugs depressing myocardial contractility (beta-blocker)
(b) noncardiogenic
1. Renal failure
2. Massive IV fluid overload
3. Hyperosmolar fluid (eg, contrast medium)
(c) neurogenic
? sympathetic venoconstriction in cerebrovascular accident, head injury, CNS tumor, postictal state
B. DECREASED COLLOID OSMOTIC PRESSURE
1. Hypoproteinemia
2. Transfusion of crystalloid fluid
3. Rapid reexpansion of lung
C. INCREASED CAPILLARY PERMEABILITY
Endothelial injury from
(a) physical trauma: parenchymal contusion, radiation therapy
(b) aspiration injury:
1. Mendelson syndrome (gastric contents)
2. Near drowning in sea water / fresh water
3. Aspiration of hypertonic contrast media
(c) inhalation injury:
1. Nitrogen dioxide = silo-filler’s disease
2. Smoke (pulmonary edema may be delayed by 24–48 hours)
3. Sulfur dioxide, hydrocarbons, carbon monoxide, beryllium, cadmium, silica, dinitrogen tetroxide, oxygen, chlorine, phosgene, ammonia, organophosphates
(d) injury via bloodstream
1. Vessel occlusion: shock (trauma, sepsis, ARDS) or emboli (air, fat, amniotic fluid, thrombus)
2. Circulating toxins: snake venom, paraquat
3. Drugs: heroin, morphine, methadone, aspirin, phenylbutazone, nitrofurantoin, chlorothiazide
4. Anaphylaxis: transfusion reaction, contrast medium reaction, penicillin
5. Hypoxia: high altitude, acute large airway obstruction
mnemonic: ABCDEFGHI – PRN
Aspiration
Burns
Chemicals
Drugs (heroin, nitrofurantoin, salicylates)
Exudative skin disorders
Fluid overload
Gram-negative shock
Heart failure
Intracranial condition
Polyarteritis nodosa
Renal disease
Near drowning
Atypical pulmonary edema = lung edema with an unusual radiologic appearance
Unusual form of pulmonary edema = lung edema from unusual causes
Increased Hydrostatic Pressure Edema
• Pulmonary capillary wedge pressure (PCWP):
= reflects left atrial pressure and correlates well with radiologic features of CHF + pulmonary venous HTN
◊ In acute CHF radiologic features are delayed in onset and resolution
√ flow inversion = “cephalization of pulmonary vessels” is ONLY seen in longstanding left heart failure, NEVER in pulmonary edema of renal failure / overhydration / low oncotic pressure
HRCT:
√ smooth interlobular septal + peribronchovascular thickening
√ ground-glass opacities in a perihilar / dependent distribution, which may progress to consolidation
√ centrilobular ground-glass nodules
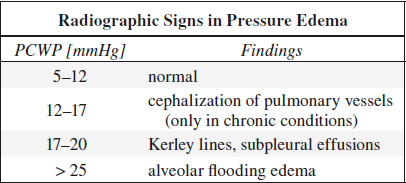
Interstitial Pulmonary Edema
= 1st phase of pressure edema with increase in quantity of extracellular fluid
Cause: increase in mean transmural arterial pressure of 15–25 mmHg
√ mild enlargement of peribronchovascular spaces
√ appearance of Kerley lines
√ subpleural effusions
√ early loss of definition of subsegmental + segmental vessels
√ progressive blurring of vessels ← central migration of edema at lobar + hilar levels
√ small peripheral vessels difficult to identify ← decrease in lung radiolucency
◊ Often marked dissociation between clinical signs + symptoms + roentgenographic evidence
◊ Nothing differentiates it from other interstitial lesions
◊ Does not necessarily develop before alveolar pulmonary edema
◊ NOT typical for bacterial pneumonia
Alveolar Flooding Edema
= 2nd phase of pressure edema
Cause: increase in mean transmural arterial pressure of > 25 mmHg ± pressure-induced damage to alveolar epithelium
√ tiny nodular / acinar areas of increased opacity
√ frank consolidation
Bat-Wing Edema (in < 10%)
= central nongravitational distribution of alveolar edema
Cause: rapidly developing severe cardiac failure (acute mitral insufficiency associated with papillary muscle rupture, massive MI, valve leaflet destruction by septic endocarditis) or renal failure
√ lung cortex spared from fluid (due to pumping effect of respiration / contractile property of alveolar septa / mucopolysaccharide-filled perivascular matrix)
Asymmetric Distribution of Pressure Edema
Cause: morphologic lung changes in COPD, hemodynamics, patient position
√ lung apices spared (= lung emphysema in heavy smokers)
√ upper + middle portions of lung spared (= end-stage TB, sarcoidosis, asbestosis)
√ predominantly RUL involvement (= mitral regurgitation refluxes preferentially into right upper pulmonary vein)
√ anteroposterior gradient on CT in recumbent position
√ unilateral edema in lateral decubitus position
Pulmonary Edema with Acute Asthma
Cause: air trapping maintains a positive intraalveolar pressure and thus decreases hydrostatic pressure gradient
Pathogenesis: associated with severity of Müller maneuver
√ heterogeneous edema ← nonuniform airway obstruction
√ peribronchial cuffing
√ ill-defined vessels
√ enlarged ill-defined hila
√ patency of narrowed airways maintained ← high negative pleural pressure in forced inspiration
Postobstructive Pulmonary Edema
Cause: following relief from an upper airway obstruction (impacted foreign body, laryngospasm, epiglottitis, strangulation)
Pathogenesis:
(a) forced inspiration causes a high negative intrathoracic pressure (Müller maneuver) and increases venous return
(b) obstruction creates high positive intrathoracic pressure that impairs development of edema
√ septal lines, peribronchial cuffing
√ central alveolar edema
√ normal heart size
Prognosis: resolution within 2–3 days
Edema with Pulmonary Embolism (< 10%)
Cause: occlusion of pulmonary arterial bed causes redirection of blood flow and hypertension in uninvolved areas
√ areas of ground-glass attenuation
√ sharply demarcated from areas of transparency distal to occluded arteries
√ associated with dilated pulmonary arteries (70%)
Edema & Pulmonary Venoocclusive Disease
Cause: organized thrombi in small veins causes an increase in peripheral resistance and hydrostatic pressure
• rapidly progressive dyspnea, orthopnea ± hemoptysis
• normal / low pulmonary capillary wedge pressure
√ enlarged pulmonary arteries
√ diffuse interstitial edema + numerous Kerley lines
√ peribronchial cuffing
√ dilated right ventricle
Permeability Edema
Heroin-induced Pulmonary Edema
Hx: overdose of opiates (almost exclusively with heroin, rarely with cocaine / “crack”)
Frequency: 15% of cases of heroin overdose
Pathophysiology: depression of medullary respiratory center leading to hypoxia + acidosis
√ widespread patchy bilateral airspace consolidations
√ ill-defined vessels + peribronchial cuffing
√ markedly asymmetric gravity-dependent distribution of edema (motionless recumbent position for hours / days)
√ resolution within 1 or 2 days in uncomplicated cases
Cx:
(1) Extensive crush injuries with associated muscle damage and ensuing renal insufficiency (from motionless recumbency)
(2) Aspiration of gastric contents
Prognosis: 10% mortality rate
Edema following Administration of Cytokines
(a) intravenous interleukin 2 (IL–2):
enhances tumoricidal activity of natural killer cells in metastatic melanoma + RCC
(b) intraarterial tumor necrosis factor:
increases production + release of IL–2
Frequency: in 75% of IL–2 therapy;
in 20% of tumor necrosis factor therapy;
in 25% of recombinant IL–2 therapy
Pathophysiology: permeability disruption of capillary endothelial cells
• 12 mmHg increase in pulmonary capillary wedge pressure (= direct toxic effect on myocardium)
√ pulmonary edema 1–5 days after start of therapy:
√ bilateral symmetric interstitial edema with thickened septal lines
√ peribronchial cuffing (75%)
√ small pleural effusions (40%)
√ NO alveolar edema (unless associated cardiac insufficiency)
High-altitude Pulmonary Edema
Predisposed: young males after rapid ascent to > 3,000 m
Cause: prolonged exposure to low partial oxygen atmospheric pressure
Pathophysiology: acute persistent hypoxia with endothelial leakage
• prodromal acute mountain sickness
• dyspnea at rest, cough with frothy pink sputum
• neurologic disturbances ← brain edema
• arterial oxygen levels as low as 38%
√ central interstitial pulmonary edema
√ peribronchial cuffing
√ ill-defined vessels
√ patchy airspace consolidation
Mixed Hydrostatic & Permeability Edema
Neurogenic Pulmonary Edema
Frequency: in up to 50% of severe brain trauma, stroke, subarachnoid hemorrhage, status epilepticus
Pathophysiology: modification in neurovegetative pathways causes sudden ↑ in pressure in pulmonary venules with reduced venous outflow
• dyspnea, tachypnea, cyanosis shortly after brain insult + rapid disappearance
√ bilateral inhomogeneous / homogeneous airspace consolidations, in 50% affecting predominantly the apices, disappearing within 1–2 days
Dx: by exclusion
DDx: fluid overload, postextubation edema
Reperfusion Pulmonary Edema
Frequency: in up to 90–100%
Cause: pulmonary thrombendarterectomy for massive pulmonary embolism / for webs and segmental stenosis
Pathophysiology: rapid increase in blood flow + pressure
• dyspnea, tachypnea, cough during the first 24–48 hours after reperfusion
√ pulmonary edema within 2 days after surgery:
√ heterogeneous airspace consolidation, predominantly in areas distal to recanalized vessels
√ random distribution in up to 50%
Reexpansion Pulmonary Edema
Cause: rapid reexpansion of a collapsed lung following evacuation of hydro-, hemo- or pneumothorax
Pathophysiology: prolonged local hypoxic event, abrupt restoration of blood flow, sudden marked increase in intrapleural pressure, diffuse alveolar damage
• frank respiratory insufficiency: cough, dyspnea, tachypnea, tachycardia, frothy pink sputum; may be asymptomatic
√ pulmonary edema within reexpanded entire lung within 1 hour (in 64%)
√ increase in severity within 24–48 hours with slow resolution over next 5–7 days
Prognosis: 20% mortality
Pulmonary Edema due to Air Embolism
Cause: usually iatrogenic complication (neurosurgical procedure in sitting position, placement / manipulation of central venous line), rare in open / closed chest trauma
Pathophysiology: embolized air bubbles cause mechanical obstruction of pulmonary microvasculature
• sudden onset of chest pain, tachypnea, dyspnea
• hypotension
√ air bubbles in right-sided cardiac chambers on echocardiography
√ interstitial edema
√ bilateral peripheral alveolar areas of increased opacity, predominantly at lung bases
Postpneumonectomy Pulmonary Edema
= life-threatening complication in early postoperative period after pneumonectomy (rare in lobectomy / lung reduction surgery)
Frequency: 2.5–5%; R > L pneumonectomy
Risk factors: excessive administration of fluid during surgery, transfusion of fresh frozen plasma, arrhythmia, marked postsurgical diuresis, low serum colloidal osmotic pressure
Pathophysiology: increased capillary hydrostatic pressure, altered capillary permeability
• marked dyspnea during first 2–3 postop days
√ ARDS-like picture
Prognosis: very high mortality rate
Pulmonary Edema after Lung Transplantation
Frequency: in up to 97% during first 3 days after surgery
Pathophysiology: tissue hypoxia, disruption of pulmonary lymphatic drainage, lung denervation
√ progressive diffuse confluent areas of increased opacity, most pronounced on postop day 5
√ return to normal 2 weeks after surgery
Unilateral Pulmonary Edema
A. IPSILATERAL = on side of preexisting abnormality
(a) filling of airways
1. Unilateral aspiration / pulmonary lavage
2. Drowned lung (bronchial obstruction)
3. Pulmonary contusion
(b) increased pulmonary venous pressure
1. Unilateral venous obstruction
2. Prolonged lateral decubitus position
(c) pulmonary arterial overload
1. Systemic artery-to-pulmonary artery shunt (Waterston, Blalock-Taussig, Pott procedure)
2. Rapid thoracentesis (rapid reexpansion)
B. CONTRALATERAL = opposite to side of abnormality
(a) pulmonary arterial obstruction
1. Congenital absence / hypoplasia of pulmonary a.
2. Unilateral arterial obstruction
3. Pulmonary thromboembolism
(b) loss of lung parenchyma
1. Swyer-James syndrome
2. Unilateral emphysema
3. Lobectomy
4. Pleural disease
C. RIGHT UPPER LOBE
PATHOGNOMONIC for mitral valve regurgitation
Pulmonary Edema with Cardiomegaly
1. Cardiogenic
2. Uremic (cardiomegaly ← pericardial effusion / hypertension)
Pulmonary Edema without Cardiomegaly
mnemonic: U DOPA
Uremia
Drugs
Overhydration
Pulmonary hemorrhage
Acute myocardial infarction, Arrhythmia
Noncardiogenic Pulmonary Edema
mnemonic: The alphabet
ARDS, Alveolar proteinosis, Aspiration, Anaphylaxis
Bleeding diathesis, Blood transfusion reaction
CNS (increased pressure, trauma, surgery, CVA, cancer)
Drowning (near), Drug reaction
Embolus (fat, thrombus)
Fluid overload, Foreign-body inhalation
Glomerulonephritis, Goodpasture syndrome, Gastrografin® aspiration
High altitude, Heroin, Hypoproteinemia
Inhalation (SO2, smoke, CO, cadmium, silica)
–
Narcotics, Nitrofurantoin
Oxygen toxicity
Pancreatitis
–
Rapid reexpansion of pneumothorax / removal of pleural effusion
–
Transfusion
Uremia
PNEUMONIA
“Classic” pneumonia pattern:
| 1. | Lobar distribution: | Streptococcus pneumoniae |
| 2. | Bulging fissure: | Klebsiella |
| 3. | Pulmonary edema: | Viral / Pneumocystis pneumonia |
| 4. | Pneumatocele: | Staphylococcus |
| 5. | Alveolar nodules: | Varicella, bronchogenic spread of TB |
Distribution:
A. SEGMENTAL / LOBAR
› Normal host: S. pneumoniae, Mycoplasma, virus
› Compromised host: S. pneumoniae
B. BRONCHOPNEUMONIA
› Normal host: Mycoplasma, virus, Streptococcus, Staphylococcus, S. pneumoniae
› Compromised host: gram-negative, Streptococcus, Staphylococcus
› Nosocomial: gram-negative, Pseudomonas, Klebsiella, Staphylococcus
› Immunosuppressed: gram-negative, Staphylococcus, Nocardia, Legionella, Aspergillus, Phycomycetes
C. EXTENSIVE BILATERAL PNEUMONIA
› Normal host: virus (eg, influenza), Legionella
› Compromised host: candidiasis, Pneumocystis, TB
D. BILATERAL LOWER LOBE PNEUMONIA
› Normal host: anaerobic (aspiration)
› Compromised host: anaerobic (aspiration)
E. PERIPHERAL PNEUMONIA
› Noninfectious eosinophilic pneumonia
Transmission:
A. Community-acquired pneumonia
Organism: viruses, S. pneumoniae, Mycoplasma
Mortality: 10%
B. Nosocomial pneumonia
[nosos, Greek = disease; kamnein, Greek = to suffer; nosocomium, Latin = hospital]
(a) gram-negative organism (> 50%): Klebsiella pneumoniae, P. aeruginosa, E. coli, Enterobacter
(b) gram-positive organism (10%): S. aureus, S. pneumoniae, H. influenzae
Complications:
1. Empyema
2. Pulmonary abscess
3. Cavitary necrosis
4. Pneumatocele
5. Pneumothorax
6. Pyopneumothorax
7. Bronchopleural fistula
Bacterial Pneumonia
Lobar Pneumonia
= ALVEOLAR PNEUMONIA
= pathogens reach peripheral air space, incite exudation of watery edema into alveolar space, centrifugal spread via small airways, pores of Kohn + Lambert into adjacent lobules + segments
√ nonsegmental sublobar consolidation
√ round pneumonia (= uniform involvement of contiguous alveoli)
(a) Streptococcus pneumoniae
(b) Klebsiella pneumoniae (more aggressive); in immunocompromised + alcoholics
(c) any pneumonia in children
(d) atypical measles
√ expansion of lobe with bulging of fissures
√ lung necrosis with cavitation
√ lack of volume loss
DDx: aspiration; pulmonary embolus
Lobular Pneumonia
= BRONCHOPNEUMONIA
= combination of interstitial + alveolar disease (injury starts in airways, involves bronchovascular bundle, spills into alveoli, which may contain edema fluid, blood, leukocytes, hyaline membranes, organisms)
Organism:
(a) Staphylococcus aureus, Pseudomonas pneumoniae: thrombosis of lobular artery branches with necrosis and cavitation
(b) Streptococcus (pneumococcus), Klebsiella, Legionnaires’ bacillus, Bacillus proteus, E. coli, anaerobes (Bacteroides + Clostridia), Nocardia, actinomycosis
(c) Mycoplasma
√ small fluffy ill-defined acinar nodules, which enlarge with time
√ lobar + segmental densities with volume loss from airway obstruction ← bronchial narrowing + mucus plugging
Atypical Bacterial Pneumonia
= bacterial infection with radiographic appearance of viral pneumonia
Organism:
(1) Mycoplasma
(2) Pertussis
(3) Chlamydia trachomatis
Gram-negative Pneumonia
In 50% cause of nosocomial necrotizing pneumonias (including staphylococcal pneumonia)
Predisposed: elderly, debilitated, diabetes, alcoholism, COPD, malignancy, bronchitis, gram-positive pneumonia, treatment with antibiotics, respirator therapy
Organism:
1. Klebsiella
2. Pseudomonas
3. E. coli
4. Proteus
5. Haemophilus
6. Legionella
√ airspace consolidation (Klebsiella)
√ spongy appearance (Pseudomonas)
√ affecting dependent lobes (poor cough reflex without clearing of bronchial tree)
√ bilateral
√ cavitation common
Cx: (1) Exudate / empyema
(2) Bronchopleural fistula
Mycotic Lung Infection
A. IN HEALTHY SUBJECTS
1. Histoplasmosis
2. Coccidioidomycosis
3. Blastomycosis
B. OPPORTUNISTIC INFECTION
1. Aspergillosis
2. Mucormycosis (phycomycosis)
3. Candidiasis
| Growth: | (a) | mycelial form |
| (b) | yeast form (depending on environment) |
Source of contamination:
(a) soil
(b) growth in moist areas (apart from Coccidioides)
(c) contaminated bird / bat excreta
Viral Lung Infection
= VIRAL PNEUMONIA = BRONCHIOLITIS = PERIBRONCHIAL PNEUMONIA = INTERSTITIAL PNEUMONIA = LOWER RESPIRATORY TRACT INFECTION
[terms used in an attempt to differentiate from airspace pneumonia]
= infection of bronchi + peribronchial tissues
Organism:
◊ The cause of infection cannot be reliably ascertained from its imaging appearance!
A. RNA VIRUSES
(a) Influenza A: more common during infancy, may lead to severe lower respiratory tract infection; mild URI in adults
1. Avian flu (H5N1 subtype): 1997 Hong Kong; 60% fatality rate
2. Swine influenza A (H1N1): 2009 in Mexico; pandemic; 1% fatality rate
(b) Parainfluenza 1–4: common cause of seasonal URI
(c) RSV = respiratory syncytial virus: most frequent viral cause of lower respiratory tract infection in infants
(d) Human metapneumovirus (HMPV): clinically indistinguishable from RSV
(e) Rubeola (measles): highly contagious; 1–36% mortality; febrile illness before / with onset of rash
(f) Enterovirus, Coxsackie virus, ECHO virus: summertime URI
(g) Human T-cell lymphotropic virus type 1 (HTLV-1): retrovirus associated with leukemia / lymphoma; transmission by sexual contact + blood transfusion + breast feeding
(h) Hantavirus (hantavirus pulmonary syndrome): 1993 in southwestern USA
(i) Coronavirus (SARS = severe acute respiratory syndrome): 2002 Guangdong Province / China; 10% mortality rate
B. DNA VIRUSES
(a) Adenovirus: associated with Swyer-James-MacLeod syndrome in children
(b) Herpes simplex virus: in immunocompromised
(c) Varicella (chickenpox)-zoster virus: common in childhood; in 10% of adults; 2–5 days after rash
(d) Cytomegalic inclusion virus: features suggestive of bronchopneumonia
(e) Epstein-Barr virus
Path: necrosis of ciliated epithelial cells, goblet cells, bronchial mucous glands with frequent involvement of peribronchial tissues + interlobular septa
Clinical syndromes:
1. (Common) cold = mild upper respiratory tract symptoms ← tonsillopharyngitis, pharyngitis, epiglottitis, sinusitis, otitis media, conjunctivitis
• sore throat, runny nose, congestion, sneezing, cough
• headache, fever (milder than for influenza)
Cause: > 200 different viruses other than influenza; Most common: rhinovirus (30–80%), coronavirus (15%), influenza virus (10–15%)
2. Influenza
• abrupt fever > 100° F (38° C), chills and sweats, headache, myalgias, malaise, fatigue, weakness
• nasal congestion, sore throat, dry persistent cough
Cause: influenza virus types A, B, C constantly changing (= antigenic drift + shift)
Viral Pneumonia in Childhood
Pathophysiology:
tracheitis, bronchitis, bronchiolitis with peribronchial infiltrates → increased secretion of mucus + constriction of bronchi → narrowing of airways → increased resistance to air flow → air trapping → increase in residual volume; injury to alveolar cells with hyaline membranes; necrosis of alveolar walls with blood, edema, fibrin, macrophages within alveoli
Age: most common cause of pneumonia in children < 5 years of age; adults have usually acquired immunity
• retractions, tachypnea, air hunger, respiratory distress
Distribution: usually bilateral
Accuracy of CXR: 92% NPV, 30% PPV
√ hyperaeration + air trapping (best indicator!):
√ depression of diaphragm on both views:
(a) projects below anterior 6th rib
(b) projects below posterior 8th/10th rib on lordotic view
(c) loss of diaphragmatic dome on lateral view
√ increase in transverse diameter of chest
√ bowing of sternum upward + outward
√ increased anteroposterior diameter on lateral view
√ “dirty chest” = peribronchial cuffing + opacification:
√ symmetric parahilar peribronchial linear densities ← patchy atelectasis
√ bronchial wall thickening
√ interstitial pattern
√ segmental + subsegmental atelectasis with frequently changing distribution ← dislodgement of mucus plugs (common):
√ wedge-shaped / triangular densities
√ airspace pattern (50%) ← hemorrhagic edema
√ pleural effusion (20%)
√ hilar adenopathy (3%)
√ striking absence of pneumatoceles, lung abscess, pneumothorax
√ radiographic resolution lags 2–3 weeks behind clinical
| Cx: | (1) Bacterial superinfection (child becomes toxic after a week of sickness, peripheral consolidations + air bronchograms + pleural effusion) |
(2) Bronchiectasis | |
(3) Unilateral hyperlucent lung, bronchiolitis obliterans |
◊ Atypical measles pneumonia does NOT show the typical radiographic findings of viral pneumonias!
Viral Pneumonia in Adulthood
Clinical groups:
(a) atypical pneumonia in healthy host
(b) viral pneumonia in immunocompromised host
Risk factors: very young & old age, malnutrition, immunologic disorders
Histo:
(a) diffuse alveolar damage (intraalveolar edema, fibrin, variable cellular infiltrate with hyaline membrane)
(b) intraalveolar hemorrhage
(c) interstitial (intrapulmonary / airway) inflammatory cell infiltration
CXR:
√ unilateral / patchy bilateral areas of consolidation
◊ Lobar consolidation is uncommon!
√ nodular opacities
√ bronchial wall thickening
√ small pleural effusion
CT:
√ disturbances of parenchymal attenuation:
√ patchy inhomogeneities = mosaic attenuation (= bronchiolar inflammation / cicatricial scarring → bronchiolar obstruction → alveolar hypoventilation):
√ area of decreased attenuation persists during expiration ← air trapping
√ lobular ground-glass opacities (= thickening of interstitium + partial filling of airspaces)
√ pulmonary consolidation:
√ patchy + poorly defined (bronchopneumonia)
√ focal + well defined (lobar pneumonia)
√ nodules < 10 mm
√ centrilobular micronodules
√ tree-in-bud of small airway disease
= dilated centrilobular bronchioles, their lumina impacted with mucus / fluid / pus
√ widespread smooth interlobular septal thickening ± crazy paving pattern
√ bronchial ± bronchiolar wall thickening
Cx: acute pneumonia with rapid progression to ARDS
Round Pneumonia
= NUMMULAR PNEUMONIA
= fairly spherical pneumonia caused by pyogenic organisms
Pathophysiology: in young children only few intraalveolar pores of Kohn + bronchoalveolar channels of Lambert have developed to allow collateral air drift
Organism: Haemophilus influenzae, Streptococcus, Pneumococcus
Age: children >> adults
• cough, chest pain, fever of ≥ 104° F (40° C)
Location: always posterior, usually in lower lobes
√ spherical infiltrate with slightly fluffy borders + air bronchogram
√ triangular infiltrate abutting a pleural surface (usually seen on lateral view)
√ rapid change in size and shape
Cavitating Pneumonia
1. Staphylococcus aureus
2. Haemophilus influenzae
3. S. pneumoniae
other gram-negative organisms (eg, Klebsiella)
Cavitating Opportunistic Infection
◊ Repeated infections in same patient are not necessarily due to same organism!
A. FUNGAL INFECTIONS
1. Aspergillosis
2. Nocardiosis
3. Mucormycosis (= phycomycosis)
B. STAPHYLOCOCCAL ABSCESS
C. TUBERCULOSIS (nummular form)
D. SEPTIC EMBOLI
1. Anaerobic organisms
DDx: Metastatic disease in carcinoma / Hodgkin lymphoma
Recurrent Pneumonia in Childhood
A. IMMUNE PROBLEM
1. Immune deficiency
2. Chronic granulomatous disease of childhood (males)
3. Alpha 1-antitrypsin deficiency
B. ASPIRATION
1. Gastroesophageal reflux
2. H-type tracheoesophageal fistula
3. Disorder of swallowing mechanism
4. Esophageal obstruction, impacted esophageal foreign body
C. UNDERLYING LUNG DISEASE
1. Sequestration
2. Bronchopulmonary dysplasia
3. Cystic fibrosis
4. Atopic asthma
5. Bronchiolitis obliterans
6. Sinusitis
7. Bronchiectasis
8. Ciliary dysmotility syndromes
9. Pulmonary foreign body
NEONATAL LUNG DISEASE
Parenchymal Lung Disease on 1st Day of Life
◊ Radiographic findings overlap!
1. Transient tachypnea of newborn
2. Respiratory distress syndrome
3. Neonatal pneumonia
4. Meconium aspiration syndrome
5. Prematurity with accelerated lung maturity (see below)
Air Leaks in Neonatal Chest
= intrathoracic extra-alveolar gas
1. Pulmonary interstitial emphysema
2. Pneumomediastinum
3. Pneumothorax
4. Gas below visceral pleura
√ gas at lung base / against fissure
5. Pneumopericardium
6. Gas embolus to cardiac chambers / blood vessels
Mediastinal Shift & Abnormal Aeration in Neonate
A. SHIFT TOWARD LUCENT LUNG
1. Congenital diaphragmatic hernia
2. Chylothorax
3. Cystic adenomatoid malformation
B. SHIFT AWAY FROM LUCENT LUNG
1. Congenital lobar emphysema
2. Persistent localized pulmonary interstitial emphysema
3. Obstruction of mainstem bronchus (by anomalous or dilated vessel / cardiac chamber)
Pulmonary Infiltrates in Neonate
mnemonic: I HEAR
Infection (pneumonia)
Hemorrhage
Edema
Aspiration
Respiratory distress syndrome
Reticulogranular Densities in Neonate
1. Respiratory distress syndrome (90%): premature infant, inadequate surfactant
2. Prematurity with Accelerated Lung Maturity (PALM)
= IMMATURE LUNG SYNDROME:
premature infant with normal surfactant ← maternal steroid therapy / intrauterine stress
√ lung granularity (= almost clear chest)
√ small thymus (stress / steroids)
3. Transient tachypnea of the newborn
4. Neonatal group-B streptococcal pneumonia
5. Idiopathic hypoglycemia
6. Congestive heart failure
7. Early pulmonary hemorrhage
8. Infant of diabetic mother
CONGENITAL LUNG ABNORMALITIES
= SEQUESTRATION SPECTRUM
A. BRONCHOPULMONARY (LUNG BUD) ANOMALY
1. Lung agenesis-hypoplasia complex
2. Congenital pulmonary airway malformation
3. Congenital lobar overinflation / emphysema
4. Bronchial atresia
5. Bronchogenic cyst
B. VASCULAR ANOMALY
1. Absence of main pulmonary artery
2. Pulmonary sling = Anomalous origin of left pulmonary a.
3. Anomalous pulmonary venous drainage
4. Pulmonary arterial / AV malformation
C. COMBINED LUNG & VASCULAR ANOMALY
1. Scimitar / hypogenetic lung / congenital pulmonary venolobar syndrome
2. Bronchopulmonary sequestration
3. Congenital pulmonary airway malformation = cystic adenomatoid malformation
Hypogenetic Lung Syndrome
= collective name for congenital underdevelopment of one / more lobes of a lung separated into 3 forms:
1. Pulmonary agenesis
= complete absence of a lobe + its bronchus
CT:
√ missing bronchus + lobe(s)
2. Pulmonary aplasia
= rudimentary bronchus ending in blind pouch + absence of parenchyma + vessels
Incidence: 1÷10,000; R÷L = 1÷1
CT:
√ absence of ipsilateral pulmonary artery
√ bronchus terminates in dilated blind pouch
√ absence of ipsilateral pulmonary tissue
3. Pulmonary hypoplasia
= completely formed but congenitally small bronchus with rudimentary parenchyma + small vessels
Developmental causes:
resulting in intrauterine compression of chest
(a) Idiopathic (rare)
(b) Extrathoracic compression (= Potter syndrome)
1. Oligohydramnios (renal agenesis, bilateral cystic renal disease, obstructive uropathy, premature rupture of membranes)
2. Fetal ascites
(c) Thoracic cage compression
1. Thoracic bone dysplasia: Ellis-van Creveld, Jeune, thanatophoric dystrophy, severe achondroplasia
2. Muscular disease
(d) Intrathoracic compression
1. Diaphragmatic defect
2. Excess pleural fluid
3. Large intrathoracic cyst / tumor
ABNORMAL LUNG PATTERN
1. Mass
= any localized density not completely bordered by fissures / pleura
2. Consolidative (alveolar) pattern
= commonly produced by filling of air spaces with fluid (transudate / exudate) / cells / other material, ALSO by alveolar collapse, airway obstruction, confluent interstitial thickening
ground glass = hazy area of increased attenuation not obscuring bronchovascular structures
consolidation = marked increase in attenuation with obliteration of underlying anatomic features
3. Interstitial pattern
4. Vascular pattern
(a) increased vessel size: CHF, pulmonary arterial hypertension, shunt vascularity, lymphangitic carcinomatosis
(b) decreased vessel size: emphysema, thromboembolism
5. Bronchial pattern
√ wall thickening: bronchitis, asthma, bronchiectasis
√ density without air bronchogram (= complete airway obstruction)
√ lucency of air trapping (= partial airway obstruction with ball-valve mechanism)
ALVEOLAR (CONSOLIDATIVE) PATTERN
Classic appearance of airspace consolidation:
mnemonic: A2BC3
√ Acinar rosettes: rounded poorly defined nodules of acinus size (6–10 mm), best seen at periphery of opacity
√ Air alveologram / bronchogram
√ Butterfly / bat-wing distribution: perihilar / bibasilar
√ Coalescent / confluent cloudlike ill-defined opacities
√ Consolidation in diffuse, perihilar / bibasilar, segmental / lobar, multifocal / lobular distribution
√ Changes occur rapidly (labile / fleeting)
HRCT:
√ poorly marginated densities within primary lobule (up to 1 cm in size)
√ rapid coalescence with neighboring lesions in segmental distribution
√ predominantly central location with sparing of subpleural zones
√ air bronchograms
Diffuse Airspace Disease
= alveoli filled with
A. INFLAMMATORY EXUDATE = “PUS”
1. Lobar pneumonia
2. Bronchopneumonia: especially gram-negative organisms
3. Unusual pneumonia
(a) viral: extensive hemorrhagic edema especially in immunocompromised patient with hematologic malignancy + transplant
(b) Pneumocystis
(c) fungal: Aspergillus, Candida, Cryptococcus, Phycomycetes
(d) tuberculosis
4. Complication of pneumonia = lung abscess
B. HEMORRHAGE = “BLOOD”
1. Trauma: contusion
2. Pulmonary embolism, thromboembolism
3. Bleeding diathesis: leukemia, hemophilia, anticoagulants, DIC, immune thrombocytopenia (ITP)
4. Vasculitis:
Wegener granulomatosis, Goodpasture syndrome, SLE, mucormycosis, aspergillosis, Rocky Mountain spotted fever, infectious mononucleosis
5. Idiopathic pulmonary hemosiderosis
6. Bleeding metastasis: eg, choriocarcinoma
C. TRANSUDATE = “WATER” = PULMONARY EDEMA / ARDS
1. Cardiac edema
3. Hypoproteinemia
4. Fluid overload
5. Renal failure
6. Radiotherapy
7. Shock
8. Toxic inhalation
9. Drug reaction
10. Drowning
11. Aspiration
12. Adult respiratory distress syndrome
D. SECRETIONS = “PROTEIN”
1. Phospholipoprotein: Pulmonary alveolar proteinosis
2. Mucus plugging
E. “CELLS”
(a) malignant: bronchioloalveolar cell carcinoma, lymphoma
(b) T4 lymphocytes: sarcoidosis
(c) Neutrophils: infection
(d) Eosinophils: eosinophilic pneumonia
(e) Plasma cells, mast cells: hypersensitivity pneumonitis
F. INTERSTITIAL DISEASE
simulating airspace disease, eg, “alveolar sarcoid”
mnemonic: AIRSPACED
Aspiration
Inhalation, Inflammatory
Renal (uremia)
Sarcoidosis
Proteinosis (alveolar)
Alveolar cell carcinoma
Congestive (CHF)
Emboli
Drug reaction, Drowning
Air-space Opacification in Trauma
A. ACUTE PHASE
1. Pulmonary contusion
2. Pulmonary laceration
3. Aspiration pneumonia
4. Atelectasis ← splinting / mucous plug
5. Pulmonary edema: cardiogenic / noncardiogenic
B. SUBACUTE PHASE (> 24 hours) add
1. Fat embolism
2. Adult respiratory distress syndrome
Localized Airspace Disease
mnemonic: 4P’s & TAIL
Pneumonia
Pulmonary edema
Pulmonary contusion
Pulmonary interstitial edema
Tuberculosis
Alveolar cell carcinoma
Infant
Lymphoma
Acute Alveolar Infiltrate
mnemonic: I 2 CHANGE FAST
Infarct
Infection
Contusion
Hemorrhage
Aspiration
Near drowning
Goodpasture syndrome
Edema
Fungus
Allergic sensitivity
Shock lung
Tuberculosis
Chronic Alveolar Infiltrate
mnemonic: STALLAG
Sarcoidosis
Tuberculosis
Alveolar cell carcinoma
Lymphoma
Lipoid pneumonia
Alveolar proteinosis
Goodpasture syndrome
CT Angiogram Sign
= homogeneous low attenuation of lung consolidation, which allows vessels to be clearly seen
1. Lobar bronchioloalveolar cell carcinoma
2. Lobar pneumonia
3. Pulmonary lymphoma
4. Extrinsic lipid pneumonia
5. Pulmonary infarction
6. Pulmonary edema
Migratory / Fleeting Pulmonary Opacities
1. Simple pulmonary eosinophilia
2. Pulmonary hemorrhage
3. Pulmonary vasculitis
4. Cryptogenic organizing pneumonia
5. Recurrent aspiration / infection
INTERSTITIAL LUNG DISEASE
= thickening of lung interstices (= interlobular septa)
A. MAJOR LYMPHATIC TRUNKS
1. Lymphangitic carcinomatosis
2. Congenital pulmonary lymphangiectasia
3. Pulmonary edema
4. Alveolar proteinosis
B. PULMONARY VEINS (↑ pulmonary venous pressure)
1. Left ventricular failure
2. Venous obstructive disease
C. SUPPORTING CONNECTIVE TISSUE NETWORK
1. Interstitial edema
2. Chronic interstitial pneumonia
3. Pneumoconioses
4. Collagen-vascular disease
5. Interstitial fibrosis
6. Amyloid
7. Tumor infiltration within connective tissue
8. Desmoplastic reaction to tumor
Path: stereotypical inflammatory response of alveolar wall to injury
(a) acute phase: fluid + inflammatory cells exude into alveolar space, mononuclear cells accumulate in edematous alveolar wall
(b) organizing phase: hyperplasia of type II pneumocytes attempt to regenerate alveolar epithelium, fibroblasts deposit collagen
(c) chronic stage: dense collagenous fibrous tissue remodels normal pulmonary architecture
Characterizing criteria:
(a) distribution:
› vertical distribution: upper / lower lung zones
› horizontal distribution : axial (core) / parenchymal (middle) / peripheral compartment
(b) volume loss
(d) interstitial lung pattern
Classification scheme:
A. Interstitial pneumonia
1. Usual interstitial pneumonia (UIP)
2. Nonspecific interstitial pneumonia (NSIP)
3. Acute interstitial pneumonia (AIP)
4. Alveolar macrophage pneumonia (AMP) = desquamative interstitial pneumonia (DIP)
5. Bronchiolitis obliterans organizing pneumonia
B. Diffuse infiltrative disease with granulomas
1. Sarcoidosis
2. Hypersensitivity pneumonitis
C. Lymphocytic interstitial pneumonia (LIP)
D. Pneumoconiosis
E. Interstitial lung disease with cysts
1. Langerhans cell histiocytosis
2. Lymphangioleiomyomatosis
F. Interstitial lung disease with interlobular septal thickening
1. Lymphangitic carcinomatosis
2. Interstitial pulmonary edema
3. Alveolar proteinosis
G. Eosinophilic syndrome
H. Pulmonary hemorrhage
I. Vasculitis
Interstitial Lung Pattern on CXR
1. LINEAR PATTERN
(a) Kerley lines = septal lines = thickened connective septa
Path: accumulation of fluid / tissue
√ Kerley A lines = relatively long fine linear shadows in upper lungs, deep within lung parenchyma radiating from hila
√ Kerley B lines = short horizontally oriented peripheral lines extending + perpendicular to pleura in costophrenic angles + retrosternal clear space
(b) reticulations
= innumerable interlacing linear opacities suggesting a mesh / network
√ Kerley C lines = fine “spider web / lacelike” polygonal opacities distributed primarily in a peripheral / subpleural location
Path: pulmonary fibrosis (lower lobes), hypersensitivity pneumonitis (upper lobes)
√ thick linear opacities in a central / perihilar distribution
Path: (a) dilated thick-walled bronchi of bronchiectasis
(b) cysts of lymphangioleiomyomatosis / tuberous sclerosis
2. NODULAR / MILIARY PATTERN
= small well-defined innumerable uniform 3–5-mm nodules with even distribution
Path: diffuse metastatic disease, infectious granulomatous disease (TB, fungal), noninfectious granulomatous disease (pneumoconioses, sarcoidosis, eosinophilic granuloma)
3. DESTRUCTIVE FORM = honeycomb lung
Signs of Acute Interstitial Disease
√ peribronchial cuffing = thickened bronchial wall + peribronchial sheath (when viewed end on)
√ thickening of interlobular fissures
√ Kerley lines
√ perihilar haze = blurring of hilar shadows
√ blurring of pulmonary vascular markings
√ increased density at lung bases
√ small pleural effusions
Signs of Chronic Interstitial Disease
◊ HRCT ~ 60% more sensitive than CXR
√ irregular visceral pleural surface
√ reticulations:
√ fine reticulations
= early potentially reversible / minimal irreversible alveolar septal abnormality
(1) Idiopathic pulmonary fibrosis (basilar predominance)
√ coarse reticulations
in 75% related to environmental disease, sarcoidosis, collagen-vascular disorders, chronic interstitial pneumonia
√ nodularity:
in 90% related to infectious / noninfectious granulomatous process, metastatic malignancy, pneumoconioses, amyloidosis
√ linearity:
(1) Cardiogenic / noncardiogenic interstitial pulmonary edema
√ symmetric linearity
(2) Lymphangitic malignancy
√ asymmetric linearity
(3) Diffuse bronchial wall disorders: cystic fibrosis, bronchiectasis, hypersensitivity asthma
√ honeycombing
= usually subpleural clustered cystic air spaces < 1 cm in diameter with thick well-defined walls set off against a background of increased lung density (end-stage lung)
Distribution of Interstitial Disease
Perihilar Interstitial Lung Disease
(a) acute rapidly changing
1. Pulmonary edema
2. Pneumocystis pneumonitis
3. Early extrinsic allergic alveolitis
(b) chronic slowly progressive
1. Lymphangitic carcinomatosis:
often unilateral, associated with adenopathy, pleural effusion
Peripheral Interstitial Lung Disease
(a) acute rapidly changing
1. Interstitial pulmonary edema with Kerley B lines (most common)
2. Active fibrosing alveolitis
(b) chronic slowly progressive
1. Secondary pulmonary hemosiderosis
Upper Interstitial Lung Disease
(a) chronic slowly progressive ± volume loss
1. Postprimary TB (common)
2. Silicosis (common)
(b) chronic slowly progressive with volume loss
1. Sarcoidosis (common)
2. Ankylosing spondylitis (rare)
3. Sulfa drugs (rare)
(c) chronic slowly progressive without volume loss
1. Extrinsic allergic alveolitis
2. Eosinophilic granuloma
3. Aspiration pneumonia
4. Postradiation pneumonitis
5. Recurrent Pneumocystis carinii pneumonia (PCP) under aerosolized pentamidine prophylaxis
Sarcoidosis
Histoplasmosis
Idiopathic
Radiation therapy
Tuberculosis (postprimary)
Chronic extrinsic alveolitis
Ankylosing spondylitis
Progressive massive fibrosis
Chronic Diffuse Infiltrative Lung Disease
= CHRONIC INTERSTITIAL LUNG DISEASE = GENERALIZED INTERSTITIAL LUNG DISEASE
Prevalence: up to 15% of pulmonary conditions
Cause: > 200 described disorders; in only 25–30% known / established etiology; 15–20 diseases comprise > 90% of cases
• dyspnea (primary complaint)
• dry basilar rales / crackles that fail to clear with coughing
CXR:
◊ Difficult to characterize due to similar findings
◊ Differentiation into alveolar + interstitial disease is unreliable as “interstitial disease” invariably involves alveoli + vice versa
√ ± nonspecific abnormality
mnemonic: HIDE FACTS
Hamman-Rich, Hemosiderosis
Infection, Irradiation, Idiopathic
Dust, Drugs
Eosinophilic granuloma, Edema
Fungal, Farmer’s lung
Aspiration (oil), Arthritis (rheumatoid, ankylosing spondylitis)
Collagen vascular disease
Tumor, TB, Tuberous sclerosis
Sarcoidosis, Scleroderma
Zonal Predilection of Chronic Diffuse Parenchymal Lung Disease (DPLD)
CHRONIC DPLD OF UPPER LUNG ZONE
= zone with higher oxygen tension and pH, but less efficient lymphatic drainage
(a) inhalational disease
1. Silicosis
2. Coal worker pneumoconiosis
3. Extrinsic allergic alveolitis
4. Aspiration pneumonia
(b) granulomatous disease
1. Sarcoidosis
2. Langerhans cell histiocytosis (EG)
3. Postprimary TB (common)
(c) others
1. Cystic fibrosis
2. Ankylosing spondylitis
3. Chronic interstitial pneumonia
4. Sulfa drugs (rare)
5. Radiation pneumonitis
6. Recurrent Pneumocystis carinii pneumonia (PCP) under aerosolized pentamidine prophylaxis
mnemonic: CASSET
Cystic fibrosis
Ankylosing spondylitis
Silicosis
Sarcoidosis
Eosinophilic granuloma
Tuberculosis, fungus
CHRONIC DPLD OF LOWER LUNG ZONE
lower lung zone = zone with greater ventilation, perfusion, and lymphatic drainage
1. Idiopathic pulmonary fibrosis: usual interstitial pneumonia (common)
2. Lymphangitic carcinomatosis
3. Collagen vascular disease: scleroderma (common)
4. Asbestosis (posterior aspect of lung base)
5. Lymphangioleiomyomatosis
6. Chronic aspiration pneumonia with fibrosis (often regional + unilateral)
mnemonic: BAD LASS RIF
Bronchiectasis
Aspiration
Dermatomyositis
Lymphangitic spread
Asbestosis
Sarcoidosis
Scleroderma
Rheumatoid arthritis
Idiopathic pulmonary fibrosis
Furadantin®
Compartmental Predilection of Chronic DPLD
A. AXIAL / CORE COMPARTMENT
= peribronchial vascular bundles + lymphatics
1. Sarcoidosis
2. Lymphangitic carcinomatosis
3. Lymphoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree