Additionally, patients are not exposed to the potentially harmful effects of ionizing radiation.
TABLE 12.1 Practical Evaluation of Pediatric Chest Wall Lesions | ||
|---|---|---|
|
TABLE 12.2 Advantages and Disadvantages of Imaging Modalities for Evaluation of Pediatric Chest Wall Lesions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 12-3 CT Protocol for Chest Wall Lesions | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
and postcontrast T1-weighted FS sequence provide information about the vascularity of soft tissue masses and vascular anomalies.
Ossification of the skeletal elements begins in utero and continues until the 25th year of life. Due to differences in muscle mass and ossification, the chest wall of infants and children is more elastic and compliant than in adults. This results in lower resting lung volumes and a less efficient respiratory mechanism that predisposes infants and young children to atelectasis. The osseous and soft tissue components gradually become stiffer with age.16
TABLE 12.4 Key Features of Common Primary Pediatric Chest Wall Lesions | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
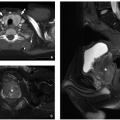
sequences may negate the need for ionizing radiation in preoperative evaluation32 (Fig. 12.6).
anterior chest wall “mass”19 (Fig. 12.8). When the etiology is not evident on physical exam, chest radiographs with a BB marker placed at the area of concern are usually sufficient. US may also provide adequate evaluation, especially for cartilaginous lesions. If CT is performed, 3D reformatted images are useful to clearly depict the prominent contour. Management is reassurance.
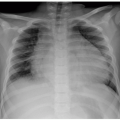
 FIGURE 12.9 Rib fusion in a 4-year-old boy. Chest radiograph shows complex scoliosis with multiple thoracic vertebral anomalies and bilateral posterior rib fusions (asterisks). |
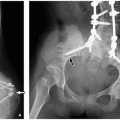
 FIGURE 12.10 Bifid rib in a 2-year-old boy referred for a chest mass. Three-dimensional reformatted CT image shows the right third bifid rib (arrow), which accounted for the palpable mass. |
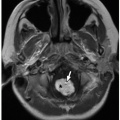
Radiographs and CT may show focal osteopenia, cortical irregularity, periosteal reaction, and contiguous soft tissue swelling. Findings are apparent on radiographs only after 7 to 10 days. Cortical irregularity and fluid adjacent to the bone and within the joint can be seen with US. Associated soft tissue changes on US include increased subcutaneous echogenicity, loss of normal soft tissue architecture, and reticular anechoic subcutaneous edema.54,55 US can be used to identify and guide drainage of superficial abscesses, which appear as hypoechoic or anechoic collections with posterior acoustic enhancement and peripheral hyperemia. Deep infection is better evaluated with CT or MRI. Although CT is superior at demonstrating osseous erosion, MRI best depicts the early changes of osteomyelitis. Abnormal marrow edema is evidenced by high T2-weighted and low T1-weighted signal intensity. Intravenous contrast may clarify regions of abscess formation (Fig. 12.15). Bone scintigraphy is sensitive for early detection but lacks anatomic detail.
TABLE 12.5 Differential Diagnosis of Aggressive-Appearing Pediatric Chest Wall Lesions | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
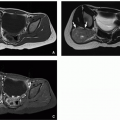
density mass. CT and MRI appearance is heterogeneous with fatty and nonfatty elements and occasionally a combination of nonenhancing cystic areas and enhancing soft tissue66 (Fig. 12.21).
involvement, which is most common in the lungs, gastrointestinal tract, and heart.68,72,73 Grossly, myofibroma/myofibromatosis is a firm white mass and variably well circumscribed. Microscopically, whorled bundles of fibroblastic cells are seen. A characteristic feature is the involvement of vessel walls (Fig. 12.24).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree