Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a chronic illness with progressive obstruction of the airways which cannot be completely reversed with medication based on bronchodilators or corticosteroids. It consists of chronic bronchitis or pulmonary emphysema.1
COPD is one of the leading causes of death worldwide. Smoking is by far the main risk factor for COPD, followed by environmental influences such as air pollution and the adverse effects of organic or inorganic dust, often in occupational settings. In rare cases, a genetic defect causes α1-antitrypsin deficiency, leading to development of pulmonary emphysema.
The main symptoms of COPD are as follows:
Chronic cough: This occurs, in particular, in the morning and generally persists over months or years.
Expectoration: There is less sputum production in pulmonary emphysema than in chronic bronchitis or bronchiectasis. If hemoptysis occurs, differential diagnosis must be carried out to exclude, for example, lung carcinoma or mycobacteriosis.
Dyspnea: At the outset, only exertional dyspnea is present. This deteriorates in the course of disease, leading to progressive immobility.
COPD is considered a systemic disease. In addition to immobility-related muscle loss, the increased breathing effort needed leads to higher energy consumption. This places greater demands on the cardiovascular system. Besides, low blood oxygen saturation levels lead to chronic myocardial ischemia. The ensuing vicious circle increases the patient’s debility, in turn leading to greater immobility.
Based on the spirometry lung function test, COPD is classified into different stages (▶Table 8.1).
Three manifest forms of COPD, which may also overlap, are distinguished: pulmonary emphysema, chronic bronchitis, and bronchiectasis.
8.1 Pulmonary Emphysema
Pulmonary emphysema is a condition involving irreversible expansion of the terminal air spaces with destruction of the alveolar walls, leading to rarefaction of the pulmonary capillary bed. The ensuing rise in pulmonary circulation resistance can cause pulmonary hypertension.
Table 8.1 Stages of chronic obstructive pulmonary disease according to the Global Initiative for Chronic Obstructive Lung Disease based on lung function parameters2 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Computed tomography (CT) is able to visualize the underlying pathomorphologic changes in greater detail than are radiographs. Likewise, classification of the different types of pulmonary emphysema as presented in the next section is based on CT morphology.
8.1.1 Emphysema on Computed Tomography
In general, destruction of the lung parenchyma with simultaneous expansion of the terminal air spaces results in lower density of the affected lung tissue on CT. While this is obvious in most cases of inhomogeneous emphysema, it may at times be difficult to identify in diffuse emphysema.
Emphysema is best visualized in a narrow lung window (e.g., width 700 HU, center -700 HU; ▶Fig. 8.1). Emphysematous portions of the lung can be displayed in a dual window with a window width of 1. The voxels whose density is above the center of the CT window appear white, and the remaining voxels are black. The center of the CT window is selected such that emphysematous lung tissue appears black and normal lung tissue white (▶Fig. 8.2). For thin slices (1-2 mm), a value of -950 HU is generally used3; for thick slices, a value of -910 HU is more suitable.4 Minimum intensity projections have proved particularly useful for detection of subtle centrilobular emphysema (see Table 1.3).
Three main types of pulmonary emphysema are distinguished depending on their distribution within the lobule:
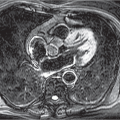
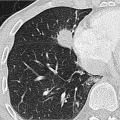
Centrilobular emphysema: This is a type of emphysema mainly caused by smoking which starts in the central portions of the acini and goes on to affect the entire lobule. Centrilobular emphysema is associated with apical predominance. On CT, rounded areas of low density with a diameter of around 1 cm can be seen. The lobular structures are preserved (▶Fig. 8.3).
The severity is graded as:
Trace if less than 0.5%.
Mild if 0.5 to 5%.
Moderate if more than 5%.
CT of the lung show visual centrilobular emphysema.1 But as the disease progresses it becomes increasingly more difficult to identify centrilobular emphysema as such on CT. This process is characterized by two newly defined subtypes of severe centrilobular emphysema1:
Confluent centrilobular emphysema: The centrilobular distribution of emphysema is no longer clearly visualized on CT. In most cases, the hypodense areas have no visible boundary (▶Fig. 8.4). Normal lung tissue can be identified between hypodense areas of the lung. Architectural distortion has not occurred yet.
Advanced destructive emphysema: This is the most advanced stage of centrilobular emphysema with homogeneously reduced density of the lung parenchyma. There is clear evidence of architectural distortion and vascular rarefaction. On radiologic imaging, this condition may be indistinguishable from panlobular emphysema (▶Fig. 8.5).
Panlobular emphysema: Panlobular emphysema leads to diffuse destruction of the lung parenchyma; the lobular structures are no longer visible. This type of emphysema is caused by a genetically mediated α1-antitrypsin deficiency. It is typically associated with basal and anterior predominance and can even be seen in young patients with this genetic predisposition (▶Fig. 8.6). Disease progression can be halted with continuous substitution therapy.
Paraseptal emphysema: Destruction of the lung parenchyma begins in the subpleural space or along the interlobular septa (▶Fig. 8.7), in particular in the apical regions. Paraseptal emphysema often causes spontaneous pneumothorax. It is graded as mild if the juxtapleural lucencies do not exceed 1-cm diameter, and as substantial in case of larger lesions.1 This type of emphysema is associated with a leptosomatic habitus which, because of the resultant effect of gravity on the lungs, is thought to give rise to particularly negative pleural pressure in the apical lung regions. This in turn increases the mechanical stress on the subpleural lung parenchyma, causing paraseptal emphysema in the apical lung regions.5,6,7 Often, mixed types of paraseptal emphysema are seen in association with the two aforementioned types. Minimal paraseptal emphysematous abnormalities are a common incidental finding even in young nonsmokers. Therefore, it is reasonable to ignore up to five small (≤1 cm) subpleural emphysematous areal at the lung apices.1,8,9
Bullous emphysema is a pulmonary emphysema subtype that is frequently observed in association with paraseptal emphysema.6 The term bulla is used to denote sharply marginated, air-containing cavities measuring more than 1 cm and enclosed in a thin wall (▶Fig. 8.8). Occasionally, bullae are also seen in the absence of recognizable emphysema. In young patients, spontaneous pneumothorax is often caused by bullae predominantly located in the apical lung regions and the apical lower lobes. The rare vanishing lung syndrome has been described in young males who develop often asymmetric giant bullous emphysema occupying at least one-third of a hemithorax. It is sometimes associated with centrilobular emphysema or bronchiectasis; smoking, cannabis abuse, and α1-antitrypsin deficiency are known predisposing factors.10,11
A special type of focal emphysema (so-called paracicatricial emphysema) is seen adjacent to areas of scarring, usually and mainly caused by sarcoidosis, silicosis, or tuberculosis.12
Quantification of pulmonary emphysema is advisable for clarification of issues such as treatment planning prior to endobronchial valve implantation or before lung volume reduction surgery as well as for drug trials. Several methodical approaches are available6:
For threshold value techniques analysis software is used to calculate the proportion of voxels of the total lung volume
with a density below a certain threshold value (usually -950 HU) (▶Fig. 8.9). Here, it is assumed that the voxels below the threshold value represent the emphysematous lung areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree