Tumors of the Lung
9.1 Hamartoma
Hamartomas (synonyms: chondroid hamartoma, chondromatous hamartoma, chondrohamartoma, hamartochondroma, chondroma) are the most common type of benign lung tumor and account for 8% of all lung neoplasms. As a malformation tumor, the hamartoma may contain fatty tissue, cartilage, epithelial tissue, and connective tissue. They are more common than leiomyomatous hamartomas.1 Two-thirds of hamartomas are asymptomatic incidental findings identified on radiographs in middle-aged patients, with men affected around two to three times more often than women. Only in around one-third of cases do hamartomas exhibit symptoms caused by bronchial obstruction, with chronic cough, hemoptysis, and fever.2
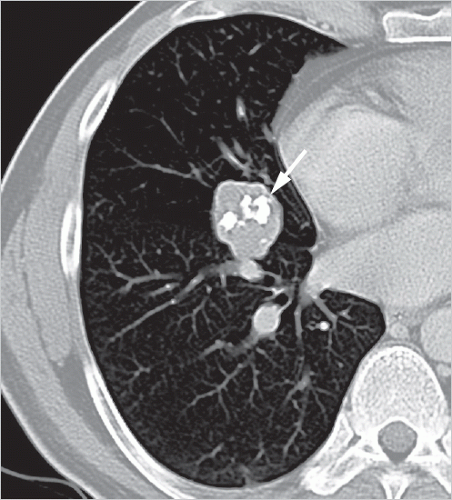
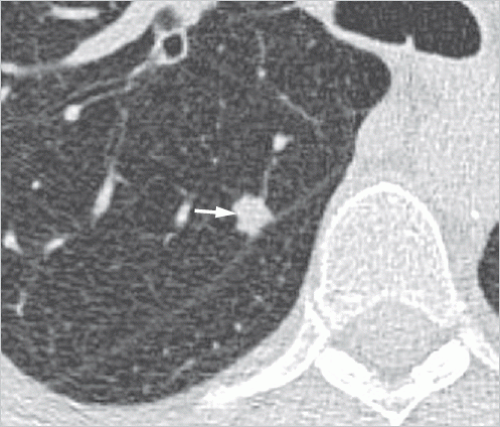
Hamartomas usually occur in the lung parenchyma, and less commonly in the endobronchial region (3-20% of cases).2 They generally manifest as peripheral, smoothly marginated, occasionally polycyclic, nodules usually measuring 1 to 2.5 cm, with a maximum diameter of 4 cm. Endobronchial hamartomas can cause inflammatory changes and atelectasis, which can lead to an atypical radiological appearance. Hamartomas containing larger amounts of chondromatous tissue typically exhibit “popcorn-like” chondroid matrix calcifications (▶Fig. 9.1); on computed tomography (CT), these are seen in 50% of the larger nodules.3 On rare occasions, diffuse calcifications are identified.4 Around half of hamartomas are found on CT to contain fat with a density of between -40 and -120 HU (▶Fig. 9.2); this is considered a reliable predictor of nodule benignity.1 Identification of fat in a smoothly marginated nodule is considered virtually pathognomonic for a hamartoma. Other fat-containing nodules are seen very rarely in the lung, in particular benign pulmonary lipomas and lipoid pneumonia.
Around half of hamartomas exhibit an identifiable growth and are usually slow growing with around 3-mm-diameter increase per year.2 Fast-growing hamartomas are observed on rare occasions and may have doubling times of less than 1 year. Often, lesion growth is an indication for surgical resection of the nodule because of a suspected malignant tumor.
A biopsy is not needed if characteristic findings have been identified on CT: smoothly marginated nodule, fat-equivalent CT density, or popcorn-like calcifications as well as no or only slow growth. Otherwise, biopsy or resection is needed, in particular in the absence of characteristic CT results or on identification of fast growth suggestive of malignancy.
Treatment is based, as far as possible, on parenchymal-sparing resection of the hamartoma. However, resection is needed only for symptomatic patients or for patients with a suspected malignant growth when biopsy cannot be performed or was inconclusive.2
9.2 Atypical Adenomatous Hyperplasia
Atypical adenomatous hyperplasia (AAH) is a preinvasive precursor lesion of adenocarcinoma of the lung. It entails
discrete proliferation of type II pneumocytes or Clara cells along the alveolar walls and occasionally also the respiratory bronchioles. The transition from AAH to adenocarcinoma in situ (AIS) is not well definable. Often, foci of AAH are found in proximity to adenocarcinomas and only discovered by chance during pathological evaluation of the resected specimens.
discrete proliferation of type II pneumocytes or Clara cells along the alveolar walls and occasionally also the respiratory bronchioles. The transition from AAH to adenocarcinoma in situ (AIS) is not well definable. Often, foci of AAH are found in proximity to adenocarcinomas and only discovered by chance during pathological evaluation of the resected specimens.
AAH cannot be identified on chest radiography since it causes too little change in the density of the lung parenchyma. On CT, it manifests, if at all visible, as a small groundglass nodule with very low density (▶Fig. 9.3). Its diameter is usually less than 5 mm but there are also reports of AAH nodules of more than 1 cm.5,6,7 The majority of the small AAH nodules detected in surgical resected specimens cannot be located even retrospectively on CT.7 AAH nodules may not change in size over several years, or they grow only very slowly.8 AAH nodules manifesting on CT as ground-glass nodules have only a very low probability of progressing to adenocarcinoma.5
AAH should be included in differential diagnosis of focal ground-glass nodules. The main reason for long-term monitoring of such lesions is that they could potentially progress to adenocarcinoma. Monitoring is required beyond the 2-year period that is sufficient for solid nodules because of the very slow development of signs of malignant growth in ground-glass nodules; hence, a follow-up of 5 years is recommended.9
9.3 Lung Cancer
Lung cancer is the leading cause of malignancy-related deaths worldwide. Several predisposing factors are known: cigarette smoking is responsible for 80% of lung cancers. Around 9 to 15% of lung cancers are attributable to occupational exposure to noxae.10 The most compelling evidence is the link between asbestos dust exposure and lung cancer; additional smoking exponentially increases the risk for lung cancer. Other physical and chemical noxae include:
Radon and other radioactive isotopes.
Quartz dust.
Chromium.
Nickel as well as nickel compounds.
Beryllium.
Arsenic.
Cadmium.
Wolfram- and cobalt-containing dusts.
Polycyclic aromatic hydrocarbons.
Dichlorodimethyl ether.
Diesel engine exhaust emissions.
A genetic predisposition is thought to be implicated especially in younger patients.
Lung cancer has a male predominance, with 2.5 times more men than women affected in developed countries. However, that gender difference has decreased in recent years, no doubt due to changing smoking habits among women. Lung cancer is predominantly diagnosed in persons aged over 60 years and only very rarely before age 40 years.
There are no early clinical symptoms. When lung cancer becomes clinically apparent, it often has already undergone lymphogenous or hematogenous spread. Hence, the diagnosis is often only made at advanced tumor stages. That explains the poor prognosis for lung cancer: the overall 5-year survival rate is below 18%.11 The prognosis is essentially better for early stage cancers, with a 5-year survival rate of 92% for stage IA1.12 The close relation between the disease stage and prognosis in combination with the lack of early symptoms suggests a potential benefit of lung cancer screening in populations at risk.
9.3.1 Classification
Based on their histology, lung cancers are classified into two main groups with considerably different prognoses and requiring different treatment approaches: small cell and nonsmall cell lung cancers. Around 80% of lung cancers are nonsmall cell lung cancers and 20% are small cell lung cancers.
Nonsmall Cell Lung Cancer
The World Health Organization Classification of Lung Tumors of 2004 recognizes five types of nonsmall cell lung cancers10:
Adenocarcinoma.
Squamous cell carcinoma.
Large cell carcinoma.
Adenosquamous carcinoma (mixed form composed of squamous cell carcinoma and adenocarcinoma).
Sarcomatoid carcinoma.
The former bronchioloalveolar carcinoma comprised a group of very heterogeneous carcinomas with highly variable prognoses. Today, those carcinomas previously known as bronchioloalveolar carcinoma are classified as various types of
adenocarcinomas (see following sections). This facilitates their assignment to treatment regimens and improves prognosis assessment.13
adenocarcinomas (see following sections). This facilitates their assignment to treatment regimens and improves prognosis assessment.13
Adenocarcinoma
Adenocarcinoma is the most common type of nonsmall cell lung cancer and occurs predominantly in the lung periphery. It also increasingly occurs in nonsmokers and younger patients without the typical risk factors, especially in women.
In the vast majority of cases, these are invasive adenocarcinomas. However, in recent years noninvasive or minimally invasive types of adenocarcinoma have drawn attention, in particular due to results from lung cancer screening trials. For that reason, a current histologic classification of adenocarcinomas is now presented below. One aspect of relevance for radiologists is the relationship between the CT morphology and histology of adenocarcinomas.14
Because of the new insights into the biologic behavior of incidentally detected small adenocarcinomas, it was necessary to revise the previous concepts used for classification of adenocarcinomas, in particular with regard to treatment and prognosis. Hence, in 2011 a new system for classification of lung adenocarcinoma, jointly compiled by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society, was published.8 As for other tumor entities, importance is now ascribed to tumor invasiveness and noninvasiveness. In an invasive carcinoma, the tumor breaches the basement membrane. Growth along the alveolar walls with wallpaper-like lining of the alveoli and preservation of aeration of the affected alveoli is termed “lepidic growth.” Recognition of a lepidic growth pattern does not exclude tumor invasiveness. Different types of adenocarcinoma are distinguished in accordance with tumor size and invasiveness (▶Fig. 9.4):
Preinvasive lesions: The term adenocarcinoma in situ refers to a cancer without an invasive component; it corresponds to one of the former types of bronchoalveolar carcinoma. The size of adenocarcinomas in situ is usually less than 2 cm, and as per their definition never more than 3 cm; they have no invasive component and are slow growing. Strictly speaking, AAH is also classified as a preinvasive lesion which is, however, a precursor lesion rather than an adenocarcinoma.
Minimally invasive adenocarcinoma (MIA): These tumors measuring up to 3 cm exhibit a lepidic growth pattern and have an invasive component of less than 5 mm.
Invasive adenocarcinoma: These adenocarcinomas exhibit a solid or lepidic growth pattern with an invasive component of over 5 mm in size. The rare tumors with a predominantly lepidic growth pattern are called lepidic predominant adenocarcinomas (LPAs) and have a better prognosis than other invasive adenocarcinomas. By contrast, adenocarcinomas classified as solid or micropapillary on histology have a particularly unfavorable prognosis.13 Invasive adenocarcinomas account for the vast majority of adenocarcinomas of the lung seen in clinical routine practice.
The various growth patterns have different imaging manifestations. In principle, a lepidic growth pattern can often be identified on imaging as an area of ground-glass density. A solid growth pattern manifests on CT either as a consolidation or as a ground-glass nodule of increasing density.5 That a nodule exhibits purely ground-glass density does not rule out an invasive carcinoma.14
Different growth patterns (see ▶Fig. 9.4) produce different imaging findings:
Solid nodule, often with spiculated margins (▶Fig. 9.5): Course spicules of more than 2 mm are correlated with a higher probability of lymphogenous metastasis and vascular invasion as well as with a poorer prognosis.5,16 Open bronchi
within the nodule—positive air bronchogram (▶Fig. 9.6)—can be identified especially in adenocarcinomas16,17,18,19 and should serve as a criterion for differential diagnosis versus benign tumor of the lung.20,21

Fig. 9.5 Pulmonary nodule. CT image. Small cell lung cancer. Coarse spicules, “corona radiata” as sign of malignancy. Distal subsegmental atelectasis, “pleural finger” (arrow).

Fig. 9.8 Part-solid nodule. CT image. Adenocarcinoma with small solid component (arrow) inside a larger ground-glass nodule.
Ground-glass nodule (▶Fig. 9.7): A focal lesion with groundglass density. It often represents an adenocarcinoma with a lepidic growth pattern, a growth pattern involving wallpaper-like, tumor-cell lining of the airspaces, which continue to be aerated.
Part-solid nodule (▶Fig. 9.8): A focal lesion with a solid and a ground-glass component; the latter is usually caused by a lepidic growth pattern (▶Fig. 9.9). This CT morphology is particularly seen in adenocarcinomas containing a mixture of solid and lepidic growth patterns (often MIA with a diameter up to 3 cm, or larger LPA).

Fig. 9.9 Lepidic predominant adenocarcinoma. CT image. Solid central tumor component (arrow) surrounded by extensive groundglass opacities (arrowheads).
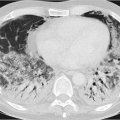
Lobar opacities (▶Fig. 9.10): Consolidations and groundglass opacities within a lobe, or in a number of lung lobes in the case of multifocal opacities, are interpreted as a characteristic manifestation of pneumonic-type lung adenocarcinoma.22 The increase in the density of the implicated lung parenchyma is due to tumor growth as well as the mucinous component which results in the areas of consolidation exhibiting lower density than that of muscle on CT.8 The pulmonary vessels traversing the tumor mass can be identified on CT as a CT angiogram sign after intravenous contrast administration.23,24,25,26
Synchronous multifocal adenocarcinomas are not uncommon; in 8 to 22% of resected pulmonary adenocarcinomas, additional lesions of the sequence AAH → AIS → invasive adenocarcinoma were identified histologically.27,28 Ground-glass nodule metastases are very rare; hence, such multiple adenocarcinomas tend to be viewed as synchronous primary tumors rather than lung metastases.8,29
Squamous Cell Carcinoma
In the past, squamous cell carcinoma was the most common type of nonsmall cell lung cancer, but today it is less common than adenocarcinoma, probably as a result of changing smoking habits. Squamous cell carcinomas are frequently located in the hilar region. They may undergo central liquefaction and then present as thick-walled cavity. Mediastinal invasion is common due to their central predominance. Some of these tumors can be quite easily surgically dissected from their surrounding structures, such as blood vessels or bronchi. Therefore, not every vascular contact of squamous cell carcinoma on staging imaging should be interpreted as vascular invasion.
Squamous cell carcinomas located at the lung periphery manifest as solid or cavitary nodules, usually with spicules. Unlike adenocarcinoma, squamous cell carcinoma does not present as subsolid nodule since it does not exhibit a lepidic growth pattern.
 Fig. 9.10 Pneumonic-type adenocarcinoma. CT image. Consolidations and ground-glass opacities in the left lower lobe, multifocal (arrow). Positive air bronchogram within the tumor (arrowhead). |
Other Nonsmall Cell Lung Cancers
A common feature of the adenosquamous, large cell, and sarcomatoid carcinomas is their peripheral predominance and nodular or mass-like appearance.
On histology, adenosquamous carcinoma is seen to contain features of both adenocarcinoma and squamous cell carcinoma. It cannot be distinguished on imaging from these two tumor entities.
The term large cell lung carcinoma is a collective term for undifferentiated, nonsmall cell lung cancers of different histologic etiology. Often, these are cancers that have undergone such profound dedifferentiation that their adeno- or squamous cell carcinoma heritage can no longer be identified on light microscopy. Neuroendocrine large cell carcinoma belongs to the group of neuroendocrine tumors, like typical and atypical carcinoids as well as small cell lung cancer.34 With respect to their differentiation at histopathology, they are classified as being between the carcinoids and small cell lung cancer.35,36 On imaging, large cell neuroendocrine carcinomas are generally seen as peripheral nodules or masses. Less commonly, they are found in central lung regions where they cause atelectasis or mucus retention.37 Peripheral large cell carcinomas usually have a smoothly marginated and lobulated, occasionally spiculated, appearance. Necrosis is very common and calcification is not uncommon.38
Sarcomatoid carcinomas are rare, predominantly peripheral, lung tumors with aggressive growth, which are relatively large at the time of diagnosis. These tumors often have extensive areas of central necrosis and tend to invade the pleura and chest wall.
Small Cell Lung Cancer
Small cell lung cancer accounts for around 20% of all lung cancers.34 Due to tumor biology, treatment and prognosis greatly differ from nonsmall cell lung cancer described above. Since it is often associated with ectopic hormone production, it frequently causes paraneoplastic syndromes, in particular Cushing syndrome or Schwartz-Bartter syndrome (syndrome of inappropriate secretion of antidiuretic hormone).39,40,41
Because of its fast growth, small cell lung cancer usually better responds to chemo- and radiotherapy than nonsmall cell lung cancer. The remission rate is around 90%, and complete remission is not uncommon.42 But, unfortunately, early recurrences after initially successful treatment are very common. In the absence of distant metastases and when disease is limited to one hemithorax (limited disease), long-term remission is observed in 20 to 25% of cases, which can be regarded as a cure. By contrast, more advanced tumor stages (extensive disease) continue to be incurable, but with palliative treatment, life expectancy can be prolonged.42
 Fig. 9.11 Radiologic appearance of peripheral and central lung cancers. Incidence of occurrence in different histologic types. |
One-fifth of all small cell lung cancers contain components of nonsmall cell lung cancer, usually of squamous cell carcinomas.43
Around 90% of small cell lung cancers are found in the central lung region and have their origin in the mucosa of the mainstem bronchus or lobar bronchi.21 Early extensive lymphogenous and hematogenous metastasis is typical. Less commonly, small cell lung cancer manifests as a peripheral nodule, typically with large hilar and mediastinal lymph node metastases by the time of diagnosis.44
9.3.2 Imaging Findings
The imaging findings of lung carcinoma are determined by its location and biologic features. Half of all lung cancers are found at the periphery. These can be directly seen on radiographs once they have reached a certain size, whereas central lung carcinomas can often only be identified through indirect signs. The typical radiologic findings are presented in ▶Fig. 9.11.
Peripheral Lung Cancer
The pulmonary nodule, defined as a rounded or irregular opacity of either solid or subsolid (part-solid or ground-glass) density, is a common presentation of small peripheral lung cancer.45,46 At times, the nodule has a lobulated appearance (▶Fig. 9.12) because of its histologic heterogeneity with variable growth rates.21 Its ill-defined or spiculated margins are known as corona radiata (see ▶Fig. 9.5) and may be caused by tumor invasion into the surrounding lung, a desmoplastic reaction of the body to the tumor, or interstitial edema.21,47,48,49 That explains the difficulties in delineating the tumor margins and determining its exact size on radiological imaging. Often, additional findings are identified. A pleural finger (see ▶Fig. 9.5) is a subsegmental atelectasis caused by the tumor and seen on CT as linear opacity extending from the nodule toward the pleura. Rigler sign (umbilical retraction sign) is the term used to denote retraction of the tumor contour at the entry point of vessels into the tumor.50 For differential diagnosis of pulmonary
nodules, please see Section 21.1. Small nodule-like lung cancers are frequently overlooked on radiographs and only diagnosed on CT. That is particularly true for ground-glass nodules or partsolid nodules. These are often incidental findings and, as small tumors, are usually asymptomatic.
nodules, please see Section 21.1. Small nodule-like lung cancers are frequently overlooked on radiographs and only diagnosed on CT. That is particularly true for ground-glass nodules or partsolid nodules. These are often incidental findings and, as small tumors, are usually asymptomatic.
A mass is a focal lesion larger than 3 cm, which is a typical appearance of larger peripheral lung cancers. The image characteristics of such lung cancers do not differ from those described in the above sections. The larger the mass, the more likely it is to impair aeration of more distal lung regions. Occasionally, adenocarcinomas contain larger ground-glass components, too.
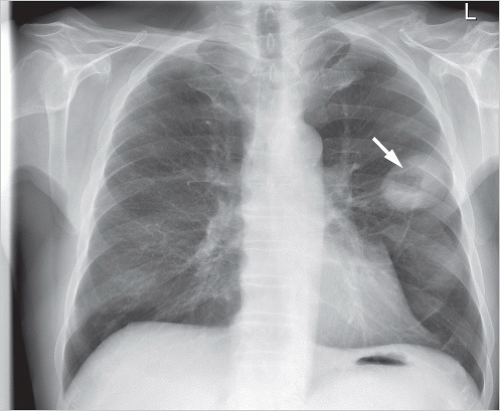
The term cavity (or cavern) is used to denote the large airfilled hollows seen at the center of pulmonary nodules or masses. On chest radiography, they manifest as ring shadows (▶Fig. 9.13). Cavities are formed by areas of central necrosis that connect with and drain into the bronchial system. Squamous cell carcinomas tend to develop thick-walled cavities.51,52 Less commonly, adenocarcinomas also have cavities which tend to be more thin-walled than those seen in squamous cell carcinomas. Cavities are uncommon in small cell lung cancer.
Adenocarcinomas, in particular of pneumonic type, may manifest as consolidation, as seen in pneumonia (see ▶Fig. 9.10). In advanced tumor stages, multiple, or even bilateral, consolidations may occasionally be seen. Often, groundglass components may be identified on CT at the periphery of the nodules. These must be distinguished from inflammatory opacities, such as poststenotic pneumonia seen in central lung cancers.
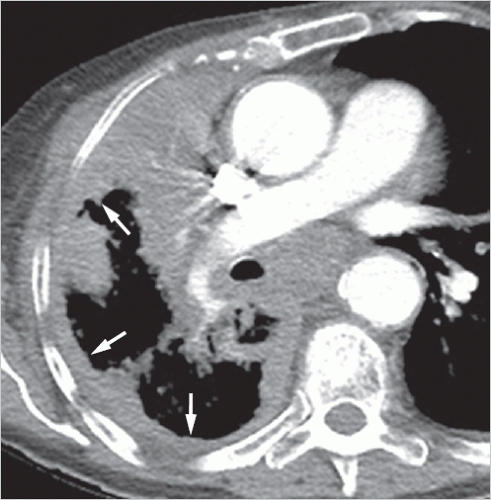
Diffuse pleural invasion is a rare manifestation of adenocarcinomas, with direct tumor spread along the pleura (▶Fig. 9.14). This causes usually irregular pleural thickening, as seen in pleural mesothelioma or pleural dissemination from other tumors.21,43 Concomitant pleural effusion may be seen but that is not necessarily the case.
Occasionally, extensive scars are seen on histology around lung cancers, which gave rise to the concept of a scar carcinoma developing in a pre-existing scar. But it is also thought that the scar may have been formed as a result of a strong desmoplastic tissue reaction to the cancer. The existence of scar carcinomas is therefore disputed.21,40,44,53,54,55
Central Lung Carcinoma
Most small cell lung cancers and squamous cell carcinomas have their origin in the central bronchial system. The latter tends to exhibit an endobronchial growth pattern and may cause atelectasis of distal peripheral lung areas. By contrast, the former tends to cause extrinsic compression of the bronchial system. Because of the central tumor localization, early invasion of the mediastinum, great blood vessels, and nerves often occurs. The phrenic nerve adjacent to the hilum is occasionally invaded by central lung carcinomas. This can be diagnosed already on a radiograph from the high-riding diaphragm. It is often difficult to distinguish between central lung cancer and their mediastinal lymph node metastases. Hence, staging problems may arise when trying to differentiate between mediastinal invasion by the primary tumor (T4) and mediastinal lymph node metastases (N2). Apart from atelectasis, bronchial and vascular stenosis has other effects: incomplete bronchial obstruction is conducive to onset of poststenotic pneumonia and, less commonly, a valve mechanism causes hyperinflation of the distal lung. Higher-grade stenosis of the central pulmonary arteries resulting from the tumor can lead to hypoperfusion of the affected lung. CT is able to directly visualize pulmonary artery stenosis; occasionally, even radiographs may demonstrate a reduced caliber of the affected pulmonary arteries and rarefaction of the pulmonary vascular bed.
Endobronchial growth of central lung carcinomas causes hypoventilation of the lung parenchyma distal to the tumor.
A tumor in the mainstem bronchus gives rise to atelectasis of the entire lung, while central tumors of the lobar bronchi cause atelectasis of an individual lung lobe. On radiographs often only the atelectasis but not the tumor can be identified. A central right upper lobe tumor may be visible within the atelectasis on the radiograph if it projects from the outer contour of the atelectatic lobe (▶Fig. 9.15). This manifestation is known as the “Golden-S sign,” first described by Ross Golden.56,57 Left-sided upper lobe atelectasis is also suggestive of a tumor even if no evidence of a tumor can be found on chest radiography. Even on CT, differentiation of a tumor from atelectasis can be challenging. Positron emission tomography (PET)-CT, which is often indicated, improves tumor within the atelectasis.
A tumor in the mainstem bronchus gives rise to atelectasis of the entire lung, while central tumors of the lobar bronchi cause atelectasis of an individual lung lobe. On radiographs often only the atelectasis but not the tumor can be identified. A central right upper lobe tumor may be visible within the atelectasis on the radiograph if it projects from the outer contour of the atelectatic lobe (▶Fig. 9.15). This manifestation is known as the “Golden-S sign,” first described by Ross Golden.56,57 Left-sided upper lobe atelectasis is also suggestive of a tumor even if no evidence of a tumor can be found on chest radiography. Even on CT, differentiation of a tumor from atelectasis can be challenging. Positron emission tomography (PET)-CT, which is often indicated, improves tumor within the atelectasis.
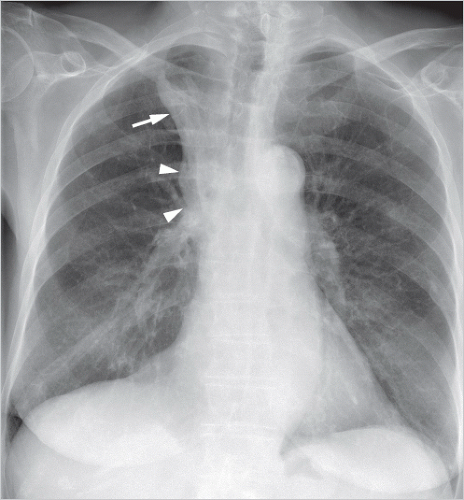
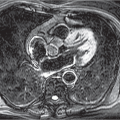
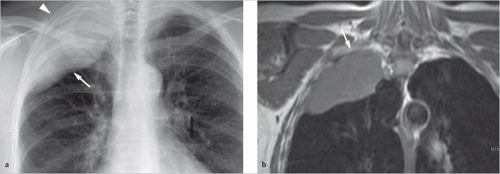 Fig. 9.16 Pancoast tumor. Lung cancer in the right upper lobe growing cranially into the soft tissues of the superior thoracic aperture. (a) Radiograph. Right apical tumor (arrow) and tumor-related opacity in the soft tissues of the upper thoracic inlet (arrowhead). (b) Coronal T1-weighted MRI image without intravenous contrast. Tumor invasion of the fatty tissues of the upper thoracic inlet (arrow). |
Other Tumor Manifestations
A pleural effusion presents in association with all the tumor manifestations described above and is an indication of the presence of pleural dissemination. But it can also be caused by atelectasis and is not malignant in such a case. The presence of pleural effusion at the time of staging should therefore not automatically be interpreted as pleural dissemination (M1a). Pleural nodules of soft-tissue density are likewise indicative of malignant pleural disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree