18.3.1 Inorganic Dust-Induced Lung Diseases (Pneumoconiosis)
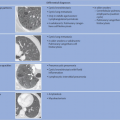
Pneumoconiosis presents as a result of a lung tissue reaction to inhaled dust particles that reach the alveoli. There is no clear-cut definition of pneumoconiosis or of how occupational lung diseases are classified as such. While the term coined in 1867 by Zenker also included organic dust, “pneumoconiosis” is currently generally understood to mean lung diseases caused by inorganic dust (▶
Table 18.1). Pneumoconiosis may be fibrogenic or nonfibrogenic (▶
Table 18.2). While berylliosis is also classified as pneumoconiosis, it occupies a special place because of its immunologic pathogenesis.
3 Beryllium is a metal used, for example, in the automobile and aircraft construction industries as well as in aerospace technology but may also be found as traces in dental alloys.
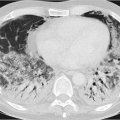
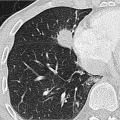
In terms of pathogenesis of pneumoconioses, it is thought that larger dust particles are eliminated by the ciliated epithelia of the tracheobronchial system or are deposited in the nasopharyngeal region. Dust particles measuring less than 5 µm in diameter and fibrogenic particles may be retained depending on the amount of dust and exposure time,
individual disposition and the integrity of the mucociliary clearance function (phagocytosis by alveolar macrophages). The mucociliary clearance function is adversely affected by cigarette smoking and toxic gases. After alveolar deposition the dust particles can trigger a chronic inflammatory reaction in the lung interstitium or be transported in the lymphatic and blood systems. Asbestos fibers can alter the pleura through pleural drift. All fibrogenic substances have the potential to cause irreversible damage to the lung parenchyma. The associated radiologic features will range from reticular (e.g., asbestosis) through reticulonodular to predominantly nodular pattern (e.g., silicosis) depending on the dust composition and severity of damage. Short-term exposure identified at an early stage often exhibits a nodular pattern with ground-glass opacity on
HRCT (which applies also to rare types of pneumoconiosis). The more chronic the exposure and disease process, the more widespread the fibrosing pattern.
Clinically, inorganic—including fibrosing—pneumoconiosis can remain silent for a very long time. This is usually followed by onset of symptoms of restrictive lung function. In particular, in silicosis obstructive ventilation disorders are also observed.
18.3.2 Organic Dust-Induced Lung Diseases
Organic dust of animal and plant origin can trigger an allergic reaction of the airways. Hypersensitivity pneumonitis is a collective term that includes a number of disease entities with similar symptoms that may be triggered by various allergens (▶
Table 18.3); for more details, please consult Section 7.1.3.
Byssinosis is a disease caused by the toxic potential of inhaled uncleaned cotton which may manifest clinically as chronic bronchitis and emphysema but is not associated with any specific radiographic features.
Pathophysiologically, hypersensitivity pneumonitis is caused by a type III and
IV immunologic response. Its pathohistology is characterized by bronchocentric lymphocytic alveolitis with granulomatous inflammation that may evolve to fibrosis.
10
Clinically, acute hypersensitivity pneumonitis may manifest with flu-like symptoms, including dyspnea and cough 6 to 8 hours after exposure. The acute variant is often self-limiting and reversible if allergen exposure is avoided early on. Persistent exposure can lead to interstitial fibrosis.
18.3.3 Acute Inhalation Toxicity
The place and extent of damage are determined by the physical state, water solubility, dose, and pH value of the noxae. The following disease entities may result:
Acute toxic tracheitis and bronchitis: Water-soluble substances (e.g., ammonia, chlorine gas, hydrochloric acid, and formaldehyde) cause damage especially to the upper respiratory tract.
Chemical irritation or toxic asthma (reactive airways dysfunction syndrome): sulfur dioxide, sulfuric acid, isocyanate, and formaldehyde can trigger acute reflex bronchoconstriction with or without reversible obstruction.
Organizing pneumonia: Following inhalational injury with high doses of, e.g., nitrogen dioxide, sulfur dioxide, ammonia, or chlorine gas, organizing pneumonia can manifest after a latency period of only up to 3 weeks.
Pulmonary edema: Substances with poor water solubility (e.g., phosgene and ozone) as well as lipophilic substances, such as nitrogen dioxide, can cause intra-alveolar edema that manifests clinically only after a dose-related latency period. Bacterial pneumonia is common because of the damage to the immune function of the alveolar macrophages.
18.3.4 Chronic Bronchitis and Asthma
There are myriad workplace-related hazardous substances that can cause chronic as well as acute irritation disorders of the airways not amenable to primary diagnostic imaging. Exceptions to that rule are chronic obstructive pulmonary diseases like chronic bronchitis and emphysema; imaging plays an important role in these disease entities when they present in the following circumstances:
In addition to the pathognomonic changes following quartz dust exposure, special attention should be paid on chest radiography, and possibly
HRCT, and to emphysema criteria since this can present as a complication of occupational chronic bronchitis.
18.3.5 Malignant Occupational Diseases of the Lung and Pleura
Malignant tumors of the lung and pleura quantitatively account for the majority of occupational cancer diseases (▶
Table 18.4). In particular, these are asbestos- and quartz dust-related lung cancers as well as asbestos-related pleural mesotheliomas. While imaging is unable to directly impute the tumor to an occupational pathogenesis, through indirect radiologic signs it can often establish a probable link to asbestosis, asbestos-induced pleural disease, or silicosis.