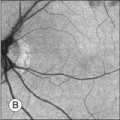
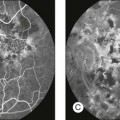
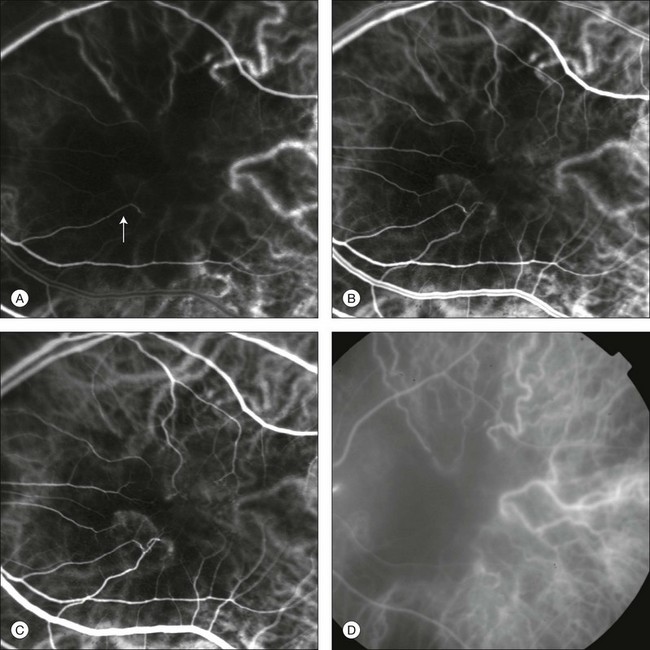
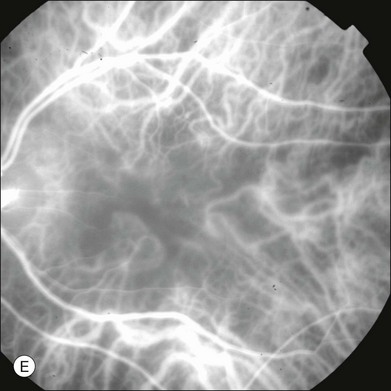
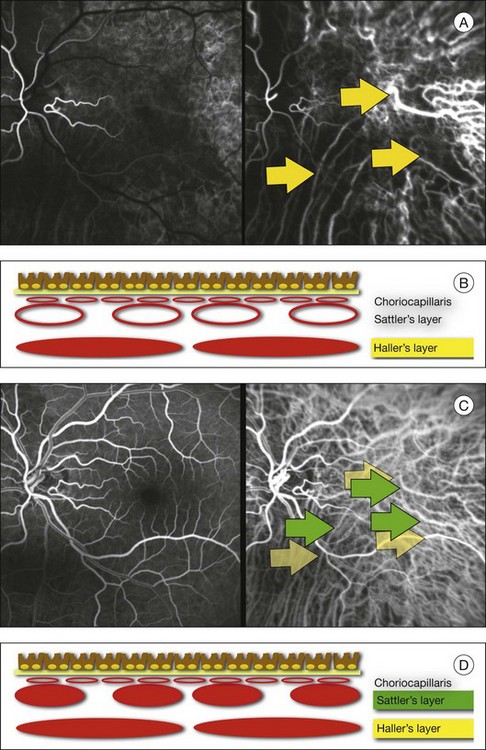
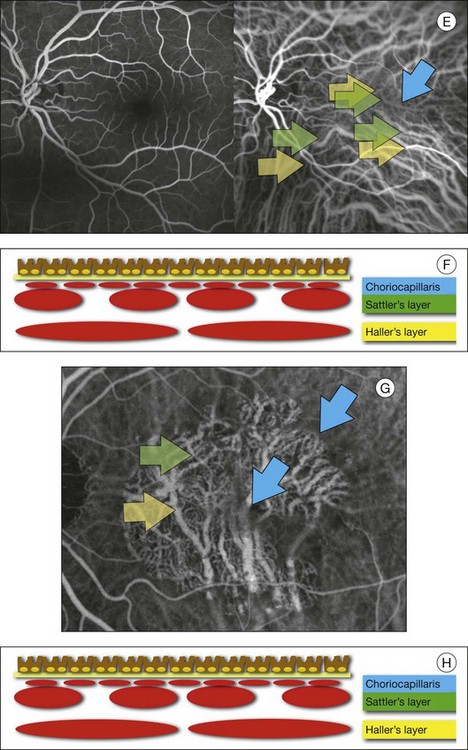
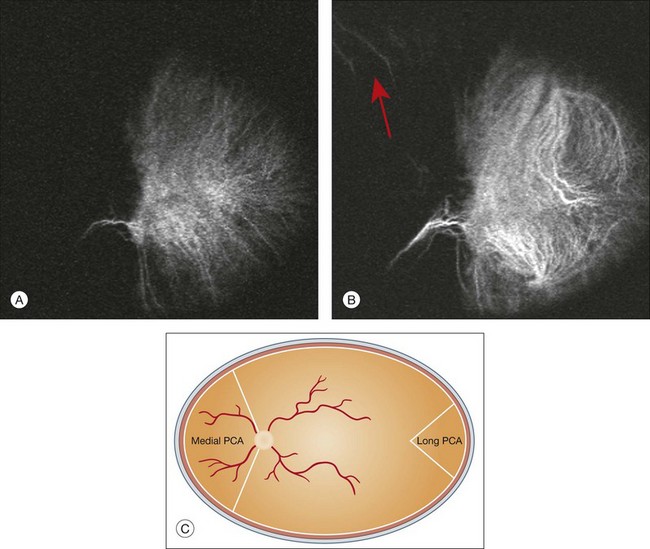
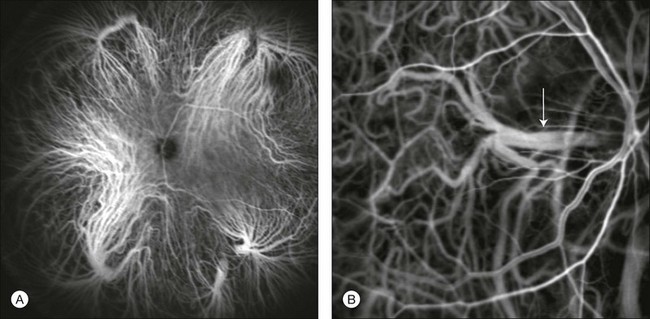
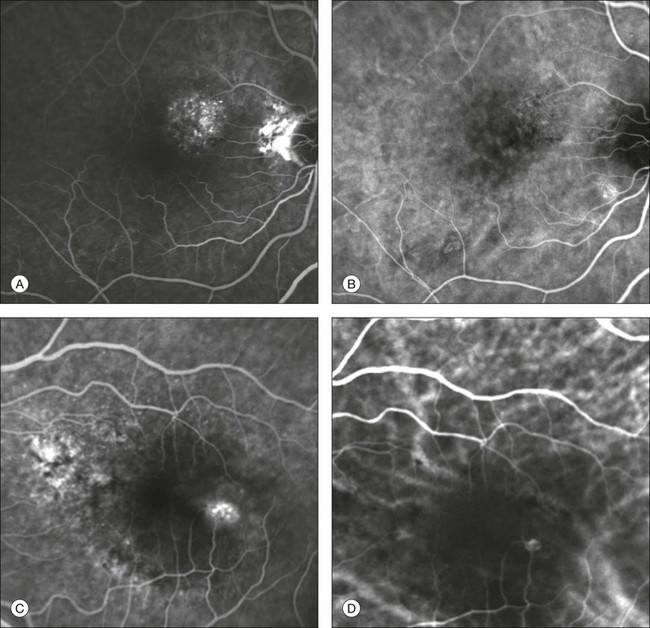
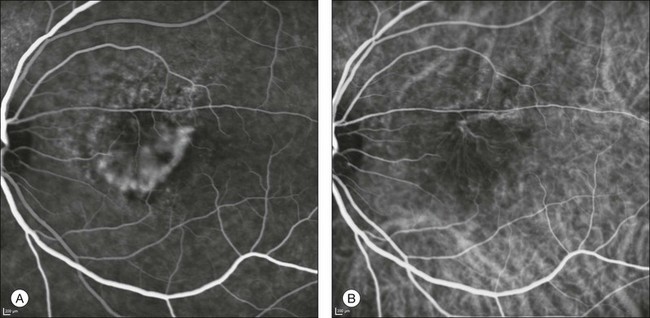
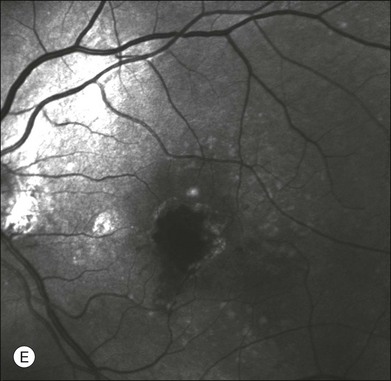
Chapter 2 Even in the era of anti-vascular endothelial growth factor (VEGF) intravitreal injections, a therapy for which accurate localization of the choroidal neovascular membrane is not as critical, ICGA, among other imaging techniques, is still extremely useful in clinical practice.1 ICGA was developed by Kodak Research Laboratories,2 on request by cardiologists to be used as an indicator of cardiac output which was not influenced by variations in blood oxygen saturation.3,4 Hepatologists subsequently began to use ICGA to evaluate hepatic blood flow5 and hepatocellular function.6 In 1969, Kogure and Choromokos first used ICGA for studying the cerebral circulation in a dog.7 The following year, Kogure et al. reported on intra-arterial ICG absorption of the choroid in monkeys.8 The first human ICG angiogram was of the carotid artery.9 In 1971, Hochheimer modified the system for ICGA by changing the color film which had previously been used to black-and-white infrared film.10 In 1972, Flower and Hochheimer performed the first intravenous ICGA to image the human choroid.11 In the following years, Flower and coworkers evaluated the potential utility of ICGA in the investigation of the normal and pathologic eye.11,12 The relatively poor fluorescence efficiency of the ICG molecule and its limited ability to produce high-resolution images on infrared film initially restricted its angiographic application. The resolution of ICGA was improved in the mid-1980s by Hayashi and de Laey, who developed improved filter combinations with sufficient sensitivity for near-infrared wavelengths.13 They were also instrumental in the transition from still-frame to dynamic imaging by introducing videoangiography.14,15 Although the sensitivity of the initial video camera system was a vast improvement over previous techniques, its inability to study individual images and the potential light toxicity using a 300-W halogen bulb restricted the duration and quality of the technique. In 1989, Destro and Puliafito16 performed ICGA using a system very similar to that described by Hayashi. In the same year, the use of the SLO for ICG videoangiography was introduced by Scheider and Schroedel.17 In 1992, Guyer et al. introduced the use of a 1024 × 1024-line digital imaging system to produce high-resolution ICGA.18 However, this system lacked flash synchronization with the video camera. Finally, Yannuzzi and coworkers described a 1024-line resolution system, which was synthesized with the appropriate flash synchronization and image storage capability, permitting high-resolution, long-duration ICGA.19 ICG is a tricarbocyanine, anionic dye. Its structural formula is 2,2′-indo-6,7,6′,7′-dibenzocarbocyanine sodium salt20 with a molecular weight of 774.96 Da.2 ICG is soluble in highly distilled water,21 even though in protein-free buffer it is difficult to obtain stable and simple ICG solutions, because of the formation of reversible dimers/polymers.2 Binding to albumin or plasma proteins improves the stability of ICG solutions.2 ICG is supplied with a solvent consisting of sterile water at pH 5.5–6.5. The final product contains 5–9.5% sodium iodine,22 to prevent recrystallization.23 ICG absorbs light in the near-infrared region of the spectrum. The maximum absorption is at 790 nm,23 while the maximum emission occurs at approximately 835 nm.24 These optical properties allow penetration through macular pigment, melanin,25 blood, and pigment. About 98% of ICG is bound to plasma protein, in particular to globulins, such as A1-lipoproteins.22 In pig plasma, lipoprotein HDL3 is the major binding protein.2 ICG is excreted by the liver,5,26 with negligible extrahepatic removal.5,27 The presumed mechanism is active27 and depends on both liver blood flow and hepatocellular function.28 ICG is excreted into the bile without metabolic process or enters the enteropathic circulation,27 through three steps: (1) uptake over the hepatotocyte sinusoidal (basolateral) membrane (Na+-mediated); (2) passage through the cell, with some role of the microfilaments and vesicular transport; and (3) excretion over the canalicular (apical) membrane.2 Rate of ICG disappearance from vascular compartment is 18–24% per minute, and after 20 minutes no more than 4% remains in the plasma.29 Plasma decay curve is initially exponential, then decelerates.26 No peripheral uptake has been described, in kidney,26 lungs, or placenta.30 ICG’s high molecular weight, in combination with the high percentage of dye bound to plasma proteins, reduces the amount of dye that exits from fenestrations in choroid vessels. This feature and its optical properties make ICG suitable for choroidal vascular network visualization. Although it has been reported that ICG can diffuse through the choroid and can accumulate in RPE cells, no dye should remain in the late phases (30–40 minutes) of the angiogram.29 ICG is considered a safe and well-tolerated dye. Its LD50 is 60 mg/kg in mice.20 Constant infusions over a 3-hour period with dosages as high as 50 mg/kg of body weight were well tolerated.27 Subcutaneous extravasation also does not produce significant local effects.26,31 Overall, the side-effect rate is low: 0.15% with mild events (nausea, vomit, sneezing, pruritus), 0.2% with moderate events (urticarial, syncope, pyrexia, nerve palsy), 0.05% with severe events (bronchospasm, laryngospasm, anaphylaxis).31 The mechanism of these various adverse side-effects is uncertain. For some, a dose-dependent pseudoallergic mechanism has been proposed,32 though there does not appear to be correlation with iodide or shellfish intolerance, suggesting that the sodium iodide component is of little significance.22,33 Nevertheless patients with a history of definite iodine allergy should not be given the dye, because of concerns for possible anaphylaxis.31 Caution should also be observed in patients affected by liver diseases29 and kidney diseases, since a 9.3% incidence of adverse reactions has been reported in dialysis patients.22,34 ICG was extensively used as a chromodiagnostic agent in the evaluation of hemodynamic changes during pregnancy.35 Nevertheless there are concerns among ophthalmologists, since the Food and Drug Administration has classified ICG as a pregnancy category C drug, meaning that adequate studies of safety have not been conducted.30 The differences between these instruments are largely related to the acquisition modality (Fig. 2.1). The light source for a digital camera is a white light with an excitation filter (640–780 nm) and a barrier filter (820–900 nm). In an SLO a laser monochromatic light is used to excite (785–790 nm) with a barrier filter at 805 nm. The laser light for an SLO system is moved on the fundus by two rotating mirrors and the image is acquired point by point. For a typical 30° image, this takes approximately 60–200 ms. The presence of a confocal aperture in SLO systems allows selective acquisition of light from a particular tissue layer (from the focal plane), and blocks the light that is coming from the surrounding tissue.36,37 Flash systems, in contrast, do not use a confocal aperture, and thus the fluorescent light returning to the camera will emanate from multiple layers. However, even for clinical confocal SLO ICG systems, the confocal aperture is much larger than one would choose for optimum z-resolution imaging. The reason for this is that fluorescence light from different depth planes (i.e., from the choroid and from the retinal vessel) needs to be imaged simultaneously for many clinical applications. Fig. 2.1 Schematic representation of the differences between digital flash fundus cameras (A), scanning laser ophthalmoscopes (SLO) (B), and confocal SLO instruments (C). In digital flash fundus cameras, white light (with or without excitation/barrier filters) is used. In SLO systems the light source is a monochromatic laser. In SLO confocal systems a pinhole aperture blocks the reflected or fluorescent light from areas outside the focal plane. These characteristics are important to recognize the different appearance of ICGA images obtained by different instruments (Fig. 2.2). Another difference is the number of images acquired per second. With SLO systems the frame rate may reach 12 images per second, thus permitting dynamic ICGA (Fig. 2.2). With digital fundus cameras the maximum rate is one frame per second. Fig. 2.2 Comparison of dynamic (six frames per second) and conventional indocyanine green angiography of a stage II retinal angiomatous proliferation lesion. A feeding retinal arteriole (A, arrow), filling of the vascular lesion, and a draining retinal vein are all characteristic features visible in the dynamic sequences (A–C). The filling sequence is missed during the first two images captured with a conventional flash fundus camera system (D, E). The concentration and preparation for intravenous injection of ICG vary with the instrument used. For fundus cameras the standard concentration is 25 mg of ICG dissolved in 5 mL solvent.38 The dosage may be increased to 50 mg in patients with poorly dilated pupils and heavy pigmentation.39 For SLOs the standard dosage is 25 mg of ICG dissolved in 3 mL, and 1 mL of the solution is injected. The solvent may be either saline alone or fluorescein sodium solution at 10–20–25% concentrations, for combined fluorescein and ICGA. Intravenous ICG injection should be rapid and immediately followed by a 5-mL saline flush. In ICGA, the early filling phase may best be correlated with the filling of different layers of the choroid. The first choroidal vessels to be filled are the ones of the deeper Haller’s layer, followed by the intermediate Sattler’s layer (Fig. 2.3). The choriocapillaris is the last layer to be filled (therefore the sequence progresses from the biggest and outermost to the smallest and innermost vessels). However, the choriocapillaris is typically quite difficult to visualize, since the resolution of the cameras is insufficient to resolve the size of its small lobular morphology. Therefore the choriocapillaris is visualized as a diffuse indistinct haze, more evident in the posterior pole and less evident in the peripheral retina (Fig. 2.3). Fig. 2.3 The filling of vessels during an indocyanine green angiogram follows a precise sequence. The first layer to be filled is Haller’s layer (A, B), followed by Sattler’s layer (C, D). After Haller’s layer and Sattler’s layer, the choriocapillaris is filled next (E, F). Simultaneous dynamic fluorescein and indocyanine green angiography (A, C, E) permits the different phases to be resolved. The choriocapillaris may be better appreciated when there are adjacent areas of focal loss, as in the example of geographic atrophy shown in panels (G) and (H). Choroidal vessels are usually first observed emanating from the posterior ciliary arteries. A well-defined watershed zone is present between the medial and lateral posterior ciliary artery (Fig. 2.4).40 Compared to fluorescein, the watershed zone is more difficult to visualize, since there is less contrast between perfused and nonperfused choroid. Fig. 2.4 A case of occlusion of the medial long posterior ciliary artery (PCA). (A) Indocyanine green angiography (ICGA) allows one to appreciate the different territories supplied by this artery and the lateral long PCA. (B) Venous phase of ICGA shows the filling of the lateral vortex veins. In the upper left area, the vortex vein is partially filled due to drainage from the iris (B, arrow). (C) Schematic representation of the different vascular territories of the two arteries. (Panel (C) modified with permission from Hayreh SS. Physiological anatomy of the choroidal vascular bed. Int Ophthalmol 1983;6:85–93.) Choroidal vortex veins are visible in the late phase of ICGA and are usually four in number (Fig. 2.5). They drain the corresponding segment of the iris, ciliary body, and choroid. Sometimes, especially in myopic eyes, a vein may be seen passing from the choroid through the sclera closely adjacent to the optic nerve head and draining into the venous plexus of the pial sheath of the optic nerve (choriovaginal vein) (Fig. 2.5).40 Fig. 2.5 Indocyanine green angiography allows visualization of the four vortex veins (A), and the choriovaginal vessels (B, arrow). In case of a thin sclera, such as in the setting of a choroidal staphyloma, extrabulbar vessels may be visualized. These can be distinguished from normal choroidal vessels because they pulsate in accordance with the heartbeat. Moreover, they change shape and position with eye movements.41,42 Exudative age-related macular degeneration (AMD) is generally classified based upon the axial location of the choroidal neovascularization (CNV). A type 1 CNV is a neovascular membrane that is located under the RPE, whereas a type 2 CNV has passed through the RPE and lies under the neurosensory retina.43 According to the Macular Photocoagulation Study, type 1 CNV is generally considered to correspond to “occult” CNV on fluorescein angiography (as defined by the Macular Photocoagulation Study), and type 2 CNV generally corresponds to “classic” CNV.44,45 More recently, type 3 CNV has been defined to be CNV with a definite intraretinal component.46 The fact that occult CNV accounts for the vast majority of the exudative complications in AMD47 explains in part why ICGA, with its ability to delineate occult CNV, has become part of standard care in exudative AMD for many clinicians.19,48,49 Nonetheless, in peer-reviewed journals, the number of articles published whose title included the terms “indocyanine green angiography (or videoangiography)” decreased between 1995 and 2010.1 One likely explanation might be the advent of anti-VEGF therapy which inaugurated a new era in the management of exudative AMD: it was the first therapy to improve the mean visual acuity of eyes treated with monthly injections of ranibizumab, regardless of whether the CNV lesion was predominantly classic50 or occult.51 Thus, the management of CNV has changed from the use of therapy for which accurate localization of the membrane was crucial (i.e., laser photocoagulation, photodynamic therapy) to the nonspecific intravitreal delivery of active and highly effective biologic drugs. Nonetheless, we would still argue that the gold-standard diagnostic procedure should be the one which best visualizes the disease (CNV) under investigation. This type of CNV is by definition under the RPE, and corresponds to the occult neovascular network on fluorescein angiography. The Macular Photocoagulation Study recognized two forms of occult CNV: (1) a fibrovascular pigment epithelial detachment (PED); and (2) a late-phase leakage of an undetermined source (LLUS).44 As for the fibrovascular PED, its prevalence may vary from 22% to 50%47–49,52 of occult CNV lesions; dynamic ICGA may delineate the presence of a neovascular network usually located along the edges of the PED49,53,54 (Fig. 2.6). Moreover, dynamic ICGA may reveal a feeder vessel that can be successfully treated with laser photocoagulation, when it is located outside the foveal region55,56 (Fig. 2.7). In case of LLUS, which may represent 36–78% of occult CNV,47,48,52 dynamic ICGA may differentiate an occult form of CNV from retinal angiomatous proliferation (RAP)52 (Fig. 2.8). Considering that one-fourth of patients with an LLUS do have a RAP,52 and that an early diagnosis of these lesions is crucial for the functional prognosis,57 the importance of an ICGA evaluation for these cases becomes readily apparent. Fig. 2.6 A pigment epithelium detachment (PED) (A, fluorescein angiography) with a well-delineated neovascular network located along the edges of the PED (B, indocyanine green angiography). Fig. 2.7 Fibrovascular pigment epithelium detachment (PED). Fluorescein angiography demonstrates occult choroidal neovascularization with PED (A). In the early phases of indocyanine green angiography (ICGA) a feeder vessel originating in the juxtapapillary area is clearly delineated (B, asterisk). Feeder vessel and draining vein are indistinguishable in the late phases of ICGA (C). Fig. 2.8 Late leakage of undetermined source (A, C). Indocyanine green angiography may clearly differentiate a subtype of occult choroidal neovascularization (B) from retinal angiomatous proliferation (D). In conclusion, ICGA faciliates a better and more complete classification of occult CNV subtypes, compared to fluorescein angiography. Of note, Yannuzzi et al.19 found that 39% of lesions classified as poorly demarcated occult lesions by fluorescein angiography were well defined by ICGA. In classic CNV, ICGA improves visualization of the fine structure of the neovascular network1 (Fig. 2.9), allowing the choroidal and retinal circulation to be distinguished. This high spatial and temporal resolution permits identification of choroidal vessels that feed into the CNV.58 In early phases, ICGA shows a dark rim which corresponds to a whitish ring on infrared imaging,59 and a discrete neovascular network surrounded by a hypocyanescent margin which is more visible after 15 minutes.60 Watzke et al.54 showed that 87% of eyes with classic choroidal neovascular membranes were hypercyanescent with distinct edges. Fig. 2.9 A case of type 2 choroidal neovascularization. In fluorescein angiography image (A), the leakage of the dye from the lesion is evident, and obscures the boundaries of the neovascular network. In indocyanine green angiography (ICGA) (B), the limits of the neovascularization are much more visible. Moreover, ICGA allows the visualization of a central feeder vessel, with a surrounding net of smaller neovessels. This ability to provide a clear delineation of the neovascular network may confer an important advantage in the era of anti-VEGF therapy. It has been reported that VEGF inhibitors were more effective in controlling immature vessels, whereas a VEGF inhibitor along with a platelet-derived growth factor (PDGF) inhibitor appeared to show a synergistic effect for controlling the growth of mature vessels.61 This is likely because pericyte recruitment is part of the maturation process in blood vessel development. Once the pericyte cell population is well established, the effectiveness of anti-VEGF agents is greatly reduced. PDGF-B is a key requirement for the recruitment of pericytes to the newly formed vessels. Mature, larger choroidal vessels may be readily differentiated from immature choroidal capillaries on ICGA (Fig. 2.10). Thus, in patients with chronic AMD or those who did not benefit from previous treatments with anti-VEGF, ICGA might better delineate a more mature stage of CNV. This has potential implications for therapeutic decision-making. Fig. 2.10 Red-free image showing intraretinal blood within a central atrophic area. A small subretinal hemorrhage is also present inferiorly (A). Corresponding fluorescein angiogram demonstrating late leakage of undetermined source along the inferior edge of the atrophic area (B). Simultaneous early-phase indocyanine green angiography (C) reveals mature, large choroidal vessel (asterisk) feeding the large net of neovascularization along the inferior edge of the central atrophy. A real chorioretinal anastomosis (arrow) is also present. Four minutes after injection (D), the draining choroidal veins are well visualized, as is the neovascular network inferiorly (D, open circles). Red-free image after a 3-month loading phase with monthly ranibizumab (E) shows an increase in size of the central retinal hemorrhage despite anti-vascular endothelial growth factor therapy. During the past decade, RAP has been labeled with a number of different terms, including “retinal vascular anomalous complex,” “retinal choroidal anastomosis,” “retinal anastomosis to the lesion,” and “chorioretinal anastomosis.” In a comprehensive article on this entity, Yannuzzi et al.62 provided evidence to support the original concept of capillaries arising within the inner half of the retina, or “retinal angiomatous proliferation,” thus suggesting the acronym of RAP as the appropriate descriptor for the disease. Subsequently, Gass et al.63 suggested a possible choroidal origin for these vessels, emanating from occult CNV and developing into an occult chorioretinal anastomosis. The new category, type 3 neovascularization, has been recently proposed46 to harmonize these conflicting theories. Type 3 lesions would encompass the following disease manifestations: (1) focal neovascular proliferation arising from the deep retinal capillary plexus (the original RAP concept); (2) intraretinal neovascular extension from an underlying occult/type I CNV; and (3) de novo breaks in Bruch’s membrane with neovascular infiltration into the retina. Whatever the origin or initial location might be, our understanding of the importance of RAP as a component of neovascular AMD has been enhanced by ICGA. The fluorescein angiographic study generally shows manifestations of occult CNV, either fibrovascular PED (Fig. 2.11) or LLUS (Fig. 2.12
Clinical Applications of Diagnostic Indocyanine Green Angiography
Introduction
History
Chemical and pharmacokinetics
Toxicity
Instrument comparison

Injection technique
Indocyanine green angiography interpretation
Exudative age-related macular degeneration
Type 1 choroidal neovascularization
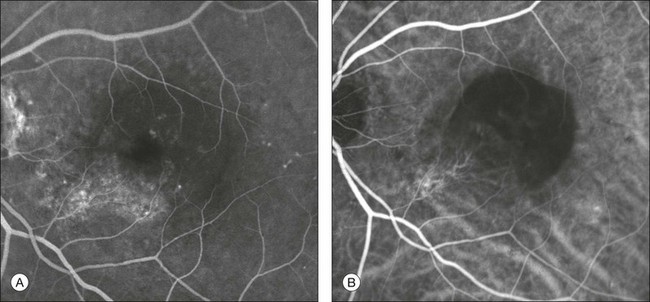

Type 2 choroidal neovascularization
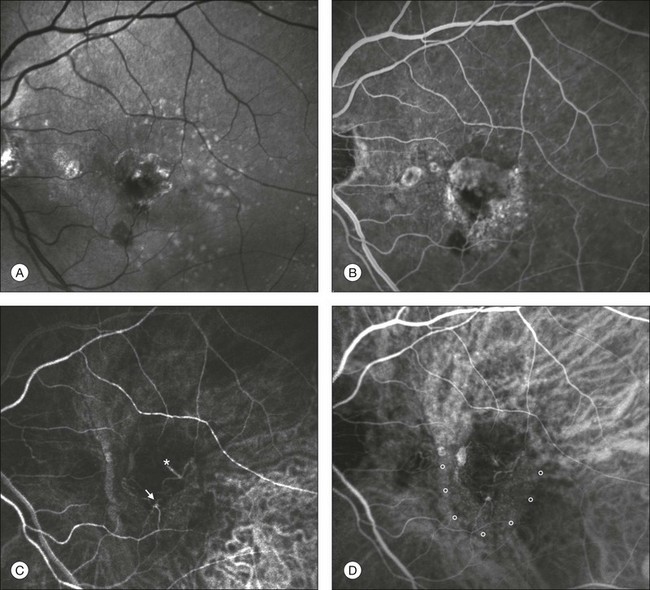
Type 3 choroidal neovascularization
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Clinical Applications of Diagnostic Indocyanine Green Angiography