The potential utility of diffusion tensor (DT) imaging in clinical practice is broad, and new applications continue to evolve as technology advances. Clinical applications of DT imaging and tractography include tissue characterization, lesion localization, and mapping of white matter tracts. DT imaging metrics are sensitive to microstructural changes associated with central nervous system disease; however, further research is needed to enhance specificity so as to facilitate more widespread clinical application. Preoperative tract mapping, with either directionally encoded color maps or tractography, provides useful information to the neurosurgeon and has been shown to improve clinical outcomes.
Key points
- •
Clinical applications of diffusion tensor (DT) imaging and tractography include tissue characterization, lesion localization, and mapping of white matter tracts.
- •
DT imaging metrics are sensitive to microstructural changes associated with central nervous system disease; however, further research is needed to enhance specificity so as to facilitate more widespread clinical application.
- •
Preoperative tract mapping, with either directionally encoded color maps or tractography, provides useful information to the neurosurgeon and has been shown to improve clinical outcomes.
Introduction
Physical Principles
Since its introduction in the mid-1980s, the applications of diffusion in magnetic resonance (MR) imaging have rapidly evolved. Diffusion-weighted (DW) and diffusion tensor (DT) imaging are based on the inherently random, thermally driven motion of molecules, known as Brownian motion, initially described by the Scottish botanist Robert Brown and mathematically characterized by Albert Einstein and others. DW and DT imaging exploit this motion of water molecules in tissues to probe the underlying microstructure, which influences molecular motion in measurable ways. By acquiring DW imaging in at least 6 noncollinear directions, a mathematical “tensor” model of diffusion can be determined that approximates the 3-dimensional (3D) diffusion profile at a given voxel with a simple ellipsoid. Because the size and shape of a 3D ellipsoid are fully specified by its diameters (so-called eigenvalues) along its 3 principal axes (major, medium, and minor), and because the orientation of the ellipsoid in 3D space is determined by those axes (eigenvectors), the complete 3D diffusion profile can be described by just a few measurable parameters. Isotropic diffusion occurs when the diffusion of water molecules occurs with equal probability in all directions (ie, the tensor ellipsoid is spherical), in contrast to anisotropic diffusion, which occurs when the structure of an underlying tissue creates a preferential axis (or axes) for molecular motion (nonspherical ellipsoid). The detailed physics of DT imaging is beyond the scope of this article, but several excellent review articles are available.
DT Imaging Quantification and Display
Whereas DT imaging of course is used to generate images that may be qualitatively interpreted, the tensor model yields several quantitative parameters reflective of tissue microstructure. The most common are the scalar metrics, mean diffusivity (MD) and fractional anisotropy (FA), which are rotationally invariant parameters reflecting the degree of (directionally averaged) diffusivity and anisotropy, respectively. MD is the tensor equivalent of the directionally averaged apparent diffusion coefficient (ADC) in common clinical use. The largest (major) eigenvalue is often called axial, longitudinal, or parallel diffusivity, as it is presumed to reflect the magnitude of diffusion parallel to axons. The average of the 2 lesser (medium and minor) eigenvalues is often called radial, transverse, or perpendicular diffusivity, as it is presumed to reflect the magnitude of diffusion perpendicular to axons (although this makes sense only under the assumption of a single population of unidirectional axons within an imaging voxel). Song and colleagues have suggested that radial diffusivity is linked to the myelin content of fiber tracts, whereas axial diffusivity is reflective of axonal integrity. However, as pointed out by Wheeler-Kingshott and Cercignani, multiple factors can affect the determination of axial and radial diffusivity such that these relationships may be less straightforward than often assumed.
Directionally encoded color (DEC) maps are commonly used to display the directionality of the major eigenvector (presumably paralleling local fiber bundles) using a red, green, and blue (RGB) color scheme to represent left-right, anterior-posterior, and superior-inferior directions, respectively. Color intensity is often weighted by an index of diffusion anisotropy (most commonly FA), yielding a convenient summary map from which the degree of anisotropy and the local fiber direction can be determined. DEC maps are particularly appealing in clinical practice because anyone familiar with normal fiber-tract anatomy can readily survey the organization of the major tracts by paging through 2-dimensional sections just as standard clinical MR images are typically viewed. Moreover, the relationship of a lesion to specific tracts in the region is often readily assessed from these maps without the need for fiber tracking, or tractography, which requires additional processing as described in the next section.
Tractography
Tractography is another approach to depicting white matter (WM) connection patterns in which mathematical algorithms are used to map the trajectories of specific fiber tracts in 3D space. These trajectories are estimated from the tensor data by starting at specified locations (known as “seed” points) and iteratively taking incremental steps in the direction of maximum diffusivity. This process is repeated until some predetermined criterion for terminating the tract has been met. The resulting tractograms may be displayed in a variety of ways using 3D computer-graphic techniques.
Most tractography algorithms estimate a single discrete trajectory for each seed-point location and many use the major eigenvector to estimate the tangent of the trajectory for a WM fiber bundle. However, additional tracking methods based on the full DT field have also been developed. Seed locations are usually defined either globally over the entire brain or in a user-specified region. Tracts are typically propagated in both forward and reverse directions until some termination criterion is met; commonly used criteria include intersecting a voxel where the anisotropy is below a specified threshold, or encountering an excessively sharp “bend” between steps along the putative tract. Tracts may be defined by constraining them to pass through 1 or more specified regions of interest.
Tractography algorithms are capable of generating anatomically plausible estimates of WM trajectories in the brain, and they have been used to depict major projection pathways (eg, pyramidal tract, internal capsule, corona radiata), commissural pathways (eg, corpus callosum, anterior commissure), and association pathways (eg, arcuate, fronto-occipital, and uncinate fasciculi). Additional quantitative tractography parameters have been proposed that take into account the rotational variance of DT imaging. These metrics include connectivity (the strength and/or likelihood of any functional connection between multiple cortical/subcortical areas) and fiber density (the number of fiber trajectories identified per voxel in a region of interest).
It must be noted that a given DT imaging–based trajectory is an imperfect model-based construct and does not (indeed cannot) directly correspond to a physical axonal fiber, given the marked discrepancy of scale between the microstructural anatomy and the spatial resolution of clinical imaging. This point is easily forgotten when admiring the aesthetic depictions of WM anatomy that tractography offers. Because of this and other limitations, tractography is most often applied qualitatively and with extreme caution in clinical practice, avoiding specific, quantitative conclusions regarding tissue integrity or character. Functional MR (fMR) imaging is considered a complementary adjunct to DT imaging, and fMR imaging activation centers can be used as seed points for tractography ( Fig. 1 ).

Terminology
As clinical tractography is still in its infancy, published reports frequently use inconsistent terminology in describing pathologically altered tracts. Such descriptive terms as “disruption,” “displacement,” “deviation,” “deformation,” “destruction,” “degeneration,” “infiltration,” “interruption,” and “splaying” are frequently used in the context of DT imaging–tractography without precise definitions. In the spirit of encouraging consistency by DT imaging–tractography investigators, as well as radiologists reporting clinical DT imaging studies, the following terminology is suggested: Deviation, Infiltration, Interruption, Degeneration, and Splaying ( Box 1 ). Although these definitions are not perfect (eg, a severely deviated or infiltrated tract might be mistaken for an interrupted one), they represent a reasonable approach given the limitations inherent to DT imaging and tractography. Note also that these definitions are not mutually exclusive (eg, a tract may be both deviated and infiltrated, deviated and interrupted, and so forth) ( Fig. 2 ).
Deviation
Any portion of tract course is altered by bulk mass effect while maintaining tract coherence, with “coherence” implying that multiple adjacent fiber trajectories follow parallel pathways or they diverge/converge in an ordered fashion. “Deviation” is preferred over “displacement” because it is more specific and informative (eg, road repairs may “displace” traffic without providing an alternative route; “deviated” implies that the flow of traffic is maintained, routed around the repairs).
Infiltration
Any portion of a tract shows significantly reduced anisotropy while retaining sufficiently ordered structure to allow its identification on directional color maps and to allow fiber tracking to proceed. Note that infiltration by tumor is not discriminated from infiltration by edema, as this cannot yet be reliably done.
Interruption
Any portion of a tract is visibly discontinuous on anisotropy-weighted DEC maps, and/or fiber tracking is discontinuous despite reasonable relaxation of termination criteria. “Reasonable” termination criteria are those that impede the generation of recognizably spurious tracts but do not necessarily penalize fiber tracking for low anisotropy, provided excessively sharp turns are adequately avoided. “Interruption” is preferred over the more pathologically definitive “destruction” or the more ambiguous “disruption” (to disrupt can mean “to break apart” or to “throw into disorder,” and these meanings would have very different implications for fiber tracts). Note also that a tract may be interrupted either partially or completely.
Degeneration
A tract characterized by significantly reduced size and/or anisotropy at a substantial distance from a lesion affecting the same neural pathway (either cortical or subcortical), such that secondary Wallerian degeneration rather than infiltration can reasonably be presumed (eg, a chronically atrophic-appearing pyramidal tract in the brainstem distal to a noninfiltrating lesion of the corona radiata).
Splaying
A tract separated by a lesion into distinct bundles deviated in different directions.

Introduction
Physical Principles
Since its introduction in the mid-1980s, the applications of diffusion in magnetic resonance (MR) imaging have rapidly evolved. Diffusion-weighted (DW) and diffusion tensor (DT) imaging are based on the inherently random, thermally driven motion of molecules, known as Brownian motion, initially described by the Scottish botanist Robert Brown and mathematically characterized by Albert Einstein and others. DW and DT imaging exploit this motion of water molecules in tissues to probe the underlying microstructure, which influences molecular motion in measurable ways. By acquiring DW imaging in at least 6 noncollinear directions, a mathematical “tensor” model of diffusion can be determined that approximates the 3-dimensional (3D) diffusion profile at a given voxel with a simple ellipsoid. Because the size and shape of a 3D ellipsoid are fully specified by its diameters (so-called eigenvalues) along its 3 principal axes (major, medium, and minor), and because the orientation of the ellipsoid in 3D space is determined by those axes (eigenvectors), the complete 3D diffusion profile can be described by just a few measurable parameters. Isotropic diffusion occurs when the diffusion of water molecules occurs with equal probability in all directions (ie, the tensor ellipsoid is spherical), in contrast to anisotropic diffusion, which occurs when the structure of an underlying tissue creates a preferential axis (or axes) for molecular motion (nonspherical ellipsoid). The detailed physics of DT imaging is beyond the scope of this article, but several excellent review articles are available.
DT Imaging Quantification and Display
Whereas DT imaging of course is used to generate images that may be qualitatively interpreted, the tensor model yields several quantitative parameters reflective of tissue microstructure. The most common are the scalar metrics, mean diffusivity (MD) and fractional anisotropy (FA), which are rotationally invariant parameters reflecting the degree of (directionally averaged) diffusivity and anisotropy, respectively. MD is the tensor equivalent of the directionally averaged apparent diffusion coefficient (ADC) in common clinical use. The largest (major) eigenvalue is often called axial, longitudinal, or parallel diffusivity, as it is presumed to reflect the magnitude of diffusion parallel to axons. The average of the 2 lesser (medium and minor) eigenvalues is often called radial, transverse, or perpendicular diffusivity, as it is presumed to reflect the magnitude of diffusion perpendicular to axons (although this makes sense only under the assumption of a single population of unidirectional axons within an imaging voxel). Song and colleagues have suggested that radial diffusivity is linked to the myelin content of fiber tracts, whereas axial diffusivity is reflective of axonal integrity. However, as pointed out by Wheeler-Kingshott and Cercignani, multiple factors can affect the determination of axial and radial diffusivity such that these relationships may be less straightforward than often assumed.
Directionally encoded color (DEC) maps are commonly used to display the directionality of the major eigenvector (presumably paralleling local fiber bundles) using a red, green, and blue (RGB) color scheme to represent left-right, anterior-posterior, and superior-inferior directions, respectively. Color intensity is often weighted by an index of diffusion anisotropy (most commonly FA), yielding a convenient summary map from which the degree of anisotropy and the local fiber direction can be determined. DEC maps are particularly appealing in clinical practice because anyone familiar with normal fiber-tract anatomy can readily survey the organization of the major tracts by paging through 2-dimensional sections just as standard clinical MR images are typically viewed. Moreover, the relationship of a lesion to specific tracts in the region is often readily assessed from these maps without the need for fiber tracking, or tractography, which requires additional processing as described in the next section.
Tractography
Tractography is another approach to depicting white matter (WM) connection patterns in which mathematical algorithms are used to map the trajectories of specific fiber tracts in 3D space. These trajectories are estimated from the tensor data by starting at specified locations (known as “seed” points) and iteratively taking incremental steps in the direction of maximum diffusivity. This process is repeated until some predetermined criterion for terminating the tract has been met. The resulting tractograms may be displayed in a variety of ways using 3D computer-graphic techniques.
Most tractography algorithms estimate a single discrete trajectory for each seed-point location and many use the major eigenvector to estimate the tangent of the trajectory for a WM fiber bundle. However, additional tracking methods based on the full DT field have also been developed. Seed locations are usually defined either globally over the entire brain or in a user-specified region. Tracts are typically propagated in both forward and reverse directions until some termination criterion is met; commonly used criteria include intersecting a voxel where the anisotropy is below a specified threshold, or encountering an excessively sharp “bend” between steps along the putative tract. Tracts may be defined by constraining them to pass through 1 or more specified regions of interest.
Tractography algorithms are capable of generating anatomically plausible estimates of WM trajectories in the brain, and they have been used to depict major projection pathways (eg, pyramidal tract, internal capsule, corona radiata), commissural pathways (eg, corpus callosum, anterior commissure), and association pathways (eg, arcuate, fronto-occipital, and uncinate fasciculi). Additional quantitative tractography parameters have been proposed that take into account the rotational variance of DT imaging. These metrics include connectivity (the strength and/or likelihood of any functional connection between multiple cortical/subcortical areas) and fiber density (the number of fiber trajectories identified per voxel in a region of interest).
It must be noted that a given DT imaging–based trajectory is an imperfect model-based construct and does not (indeed cannot) directly correspond to a physical axonal fiber, given the marked discrepancy of scale between the microstructural anatomy and the spatial resolution of clinical imaging. This point is easily forgotten when admiring the aesthetic depictions of WM anatomy that tractography offers. Because of this and other limitations, tractography is most often applied qualitatively and with extreme caution in clinical practice, avoiding specific, quantitative conclusions regarding tissue integrity or character. Functional MR (fMR) imaging is considered a complementary adjunct to DT imaging, and fMR imaging activation centers can be used as seed points for tractography ( Fig. 1 ).
Terminology
As clinical tractography is still in its infancy, published reports frequently use inconsistent terminology in describing pathologically altered tracts. Such descriptive terms as “disruption,” “displacement,” “deviation,” “deformation,” “destruction,” “degeneration,” “infiltration,” “interruption,” and “splaying” are frequently used in the context of DT imaging–tractography without precise definitions. In the spirit of encouraging consistency by DT imaging–tractography investigators, as well as radiologists reporting clinical DT imaging studies, the following terminology is suggested: Deviation, Infiltration, Interruption, Degeneration, and Splaying ( Box 1 ). Although these definitions are not perfect (eg, a severely deviated or infiltrated tract might be mistaken for an interrupted one), they represent a reasonable approach given the limitations inherent to DT imaging and tractography. Note also that these definitions are not mutually exclusive (eg, a tract may be both deviated and infiltrated, deviated and interrupted, and so forth) ( Fig. 2 ).
Deviation
Any portion of tract course is altered by bulk mass effect while maintaining tract coherence, with “coherence” implying that multiple adjacent fiber trajectories follow parallel pathways or they diverge/converge in an ordered fashion. “Deviation” is preferred over “displacement” because it is more specific and informative (eg, road repairs may “displace” traffic without providing an alternative route; “deviated” implies that the flow of traffic is maintained, routed around the repairs).
Infiltration
Any portion of a tract shows significantly reduced anisotropy while retaining sufficiently ordered structure to allow its identification on directional color maps and to allow fiber tracking to proceed. Note that infiltration by tumor is not discriminated from infiltration by edema, as this cannot yet be reliably done.
Interruption
Any portion of a tract is visibly discontinuous on anisotropy-weighted DEC maps, and/or fiber tracking is discontinuous despite reasonable relaxation of termination criteria. “Reasonable” termination criteria are those that impede the generation of recognizably spurious tracts but do not necessarily penalize fiber tracking for low anisotropy, provided excessively sharp turns are adequately avoided. “Interruption” is preferred over the more pathologically definitive “destruction” or the more ambiguous “disruption” (to disrupt can mean “to break apart” or to “throw into disorder,” and these meanings would have very different implications for fiber tracts). Note also that a tract may be interrupted either partially or completely.
Degeneration
A tract characterized by significantly reduced size and/or anisotropy at a substantial distance from a lesion affecting the same neural pathway (either cortical or subcortical), such that secondary Wallerian degeneration rather than infiltration can reasonably be presumed (eg, a chronically atrophic-appearing pyramidal tract in the brainstem distal to a noninfiltrating lesion of the corona radiata).
Splaying
A tract separated by a lesion into distinct bundles deviated in different directions.
Clinical applications
As already noted, DT imaging can exploit the magnitude and directionality of diffusion to provide exquisite noninvasive characterization of tissue architecture that is not feasible with standard MR imaging. Broadly categorized, clinical applications of DT imaging include: tissue characterization (eg, estimating the histology, grade, or margins of a neoplasm); lesion localization (eg, determining the specific anatomic fiber tract involved by an underlying pathologic condition); and tract mapping (eg, preoperative mapping of a tract deviated by a space-occupying mass).
Tissue characterization is most often addressed using scalar metrics (most commonly MD, ADC, and FA) on a voxel-wise basis. Multiple studies have shown these parameters to be more sensitive to abnormalities than conventional MR imaging, based on changes found in the so-called normal-appearing white matter (NAWM). Unfortunately, this high sensitivity is accompanied by low specificity, particularly in the case of FA. Thus, although the common DT imaging metrics might have some appeal as imaging end points in clinical trials (based on their sensitivity to subclinical pathologic changes), their routine clinical utility currently is quite limited by their low specificity. Recent efforts to derive greater pathologic specificity from DT imaging parameters have focused on directionally specific (eg, axial and radial) diffusivities and other features of the diffusion tensor, but the clinical role of these emerging techniques is not yet defined. Lesion localization and tract mapping are more straightforward applications of DT imaging and tractography, a few anatomic controversies notwithstanding. The ability to localize lesions and describe their relationship to specific tracts on imaging has obvious importance to patient management.
Neoplasm
The interrogation of neoplasms has become one of the most frequent clinical applications of DT imaging and tractography. Many studies have evaluated the utility of scalar diffusion metrics (eg, MD, FA) in neoplastic tissue characterization, such as for estimating tumor type and grade or discriminating tumor infiltration from peritumoral edema. The inconsistent results of these studies overall make it difficult to apply quantitative DT imaging indices to these questions in routine clinical practice. Of all the potential relationships between DT imaging metrics and tissue properties that have been considered, the one supported by the most evidence is an inverse correlation between tumor cellularity and MD (or ADC). This relationship may have clinical utility in identifying tumors known for high cell density, such as lymphoma and medulloblastoma. However, other factors contribute to MD/ADC values such that reliable tumor identification before biopsy is usually not possible.
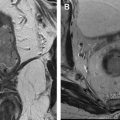
A more practical and frequently used application of DT imaging is to lesion localization and tract mapping ( Fig. 3 ). Preoperative tractography can provide confirmation that a tumor-deviated tract remains intact and can potentially facilitate preservation of the tract during resection ( Fig. 4 ). This application is probably the best known to date, although studies clearly attributing improved clinical outcomes to DT imaging are still relatively few. In one of the largest published series to date, patients with high-grade gliomas survived an average of 7 months longer and were more functional postoperatively when DT imaging was added to standard neurosurgical navigation procedures. Intraoperative DT imaging with tractography has also shown promise in monitoring the shift of WM tracts during resection. The advantages of DT imaging as a technique that is complementary to preoperative fMR imaging were demonstrated by Ulmer and colleagues, who identified twice as many functional systems near tumor margins when DT imaging and fMR imaging were combined in preoperative tumor planning, relative to fMR imaging alone.
Ischemic Stroke
Conventional DW imaging has become an invaluable tool in the evaluation of ischemic stroke, owing to its excellent sensitivity and ability to depict the infarct volume. DT imaging is capable of providing additional tissue characterization of stroke (eg, improved temporal evaluation of ischemia). In the acute phase of stroke, the MD is noted to initially decrease and the FA to increase. In the subacute phase, the MD will normalize while the FA begins to decrease. Subsequently, in the chronic phase of stroke there is an increase in MD and the FA continues to decline. Some investigators have also suggested that DT imaging may be more sensitive than conventional DW imaging in differentiating ischemia between gray matter and WM ; however, others have demonstrated conflicting results. Following stroke, secondary (Wallerian) degeneration of WM tracts has been shown to correlate with the degree of motor dysfunction ; tractography allows lesion localization (eg, distance between ischemic tissue and WM tracts) as well as tract mapping that can depict Wallerian degeneration, at times substantially better than conventional MR imaging. This technique has also been used to monitor reorganization of the WM architecture following stroke management.
Epilepsy
Epilepsy is a chronic neurologic disorder, classified as generalized or partial (localized), in which abnormal or excessive neuronal activity results in an increased risk of recurrent seizures. There are many potential causes of epilepsy including trauma, stroke, tumors, and congenital malformations. In temporal lobe epilepsy, a form of localized seizures, patients are frequently treated with anterior temporal lobectomy; a potential complication of this procedure is visual field deficits resulting from injury to the Meyer loop, the fibers of which have variable anterior extent. Tractography has been demonstrated to be feasible in depicting the optic tracts (including the Meyer loop), which may improve surgical outcome ( Fig. 5 ). Scalar DT imaging metrics have also been used to lateralize seizure foci. In patients with mesial temporal sclerosis, MD is noted to be increased in the affected hippocampal formation. The ability to lateralize the seizure focus has also been demonstrated in patients with unremarkable conventional MR imaging. Additional studies have revealed alterations of anisotropy (eg, decreased FA) in normal-appearing WM beyond an identified seizure focus.
Demyelinating Disease
Demyelinating diseases are a group of disorders in which there is loss of myelin as the sequela of inflammatory, autoimmune, infectious, nutritional, iatrogenic, toxic, or vascular causes. Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, and a major cause of chronic neurologic disability in the young and middle-aged. Unfortunately the correlation between conventional MR imaging findings and clinical disability in MS is notoriously poor; this is due, at least in part, to the lack of functional specificity in standard estimates of disease burden (eg, the total number or volume of lesions on T1-/T2-weighted images). DT imaging has the potential to improve the characterization of demyelinating plaques. Studies using scalar metrics have revealed demyelinating lesions to have increased MD and decreased FA, and FA changes extending significantly beyond lesion margins visible on conventional MR imaging. In a widely cited study in a mouse retinal nerve ischemia model, Song and colleagues suggested that axial and radial diffusivities are relatively specific for axonal and myelin degeneration, respectively; however, this relationship is unlikely to be as straightforward as is commonly assumed. Several studies have demonstrated that DT imaging reveals alterations in the diffusivity and anisotropy of NAWM. For example, Werring and colleagues demonstrated that acute demyelinating lesions are preceded by subtle diffusion changes in NAWM. These studies suggest that DT imaging may provide more meaningful imaging markers of demyelinating disease than conventional imaging, and thus enable more accurate and timely monitoring of disease progression and therapeutic effects. Lesion localization with tractography has also been shown to enable more functionally specific estimates of disease burden (“importance sampling” ), which better correlates with clinical disability ( Fig. 6 ).
Dementia
Dementia is a group of disorders in which there is cognitive decline that significantly affects activities of daily living through alterations of memory, language, visuospatial function, or executive function. There are many potential causes, including neurodegenerative, vascular, toxic, nutritional, and infectious etiology. A frequent application of DT imaging is for tissue characterization of patients with Alzheimer disease (AD), the most common of the dementias, and in mild cognitive impairment (MCI), considered a cognitive state intermediate between normal cognition and dementia. Studies have shown the effects of AD to be most pronounced in the medial temporal lobes, measurable as decreased FA and increased MD in hippocampus and adjacent gray matter. Higher MD is also observable in widespread portions of frontal, lateral and medial parietal, and lateral temporal cortices. DT imaging indices have also allowed greater understanding of the effects of AD in the commissural (eg, corpus callosum) and association (eg, cingulum and inferior fronto-occipital/uncinate/superior longitudinal fasciculi) WM tracts that connect these regions ( Fig. 7 ). In addition, tractography has been shown to be feasible in characterizing association fibers (eg, cingulum) that may play a role in the progressive impairment in AD patients. A meta-analysis performed by Sexton and colleagues revealed similar degeneration in patients with MCI, albeit with a reduced extent of differences.