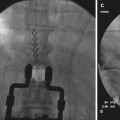
Fig. 46.1
Fluoroscopic anterior-posterior (AP) radiograph of thoracic spine with appropriately positioned two octrode leads midline and with the tips positioned at T5. This is the most frequent lead positioning when used for the SCS in painful gastrointestinal disorders
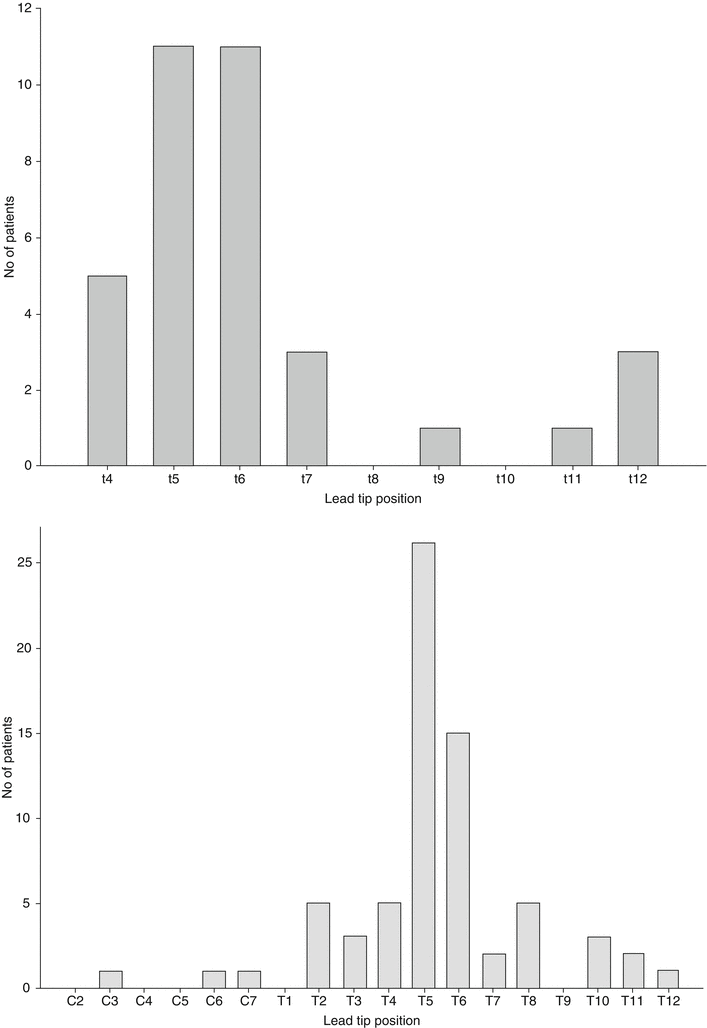
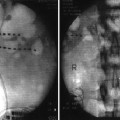
Fig. 46.2
Two graphs below illustrate distribution of the lead tip positions when SCS used for treatment of chronic visceral hyperalgesia. Graphs shown are from two published studies: (a) a retrospective larger case series on 35 patients with various causes of abdominal pain and (b) survey that collected data on 70 SCS cases. More than two-thirds of the patients were able to achieve optimal paresthesias to cover the area of abdominal pain when leads were positioned midline and up to T5 or T6 vertebral level (With permission from Pain Medicine [72, 77])
Most recently reported was a clinical experience using SCS in 30 patients with chronic pancreatitis [78]. Patient population was somewhat different, as there were 9 out of 30 patients with previous alcohol or opioid abuse. Similar SCS lead placement was required to achieve appropriate paresthesias (T5 (N = 10) or T6 (N = 10)). Twenty-four patients (80 %) had >50 % pain relief during the trial. Improvements in VAS pain scores were substantial: from 8 ± 1.6 to 3.6 ± 2 cm at 1 year (same as decrease in opioid use from 165 ± 120 to 48.6 ± 58 mg of morphine equivalents). SCS was very useful therapeutic option for >70 % of trialed patients with severe visceral pain from chronic pancreatitis [78].
Conclusion
When SCS is used in the animal models of colorectal distension and irritant-induced colonic sensitization, data suggest that the SCS may suppress visceromotor reflex in rats [65]. Recent study results suggest that SCS may be a very useful therapeutic option when trialed in patients with various chronic visceral pain conditions [72, 77]. In order to elucidate mechanisms behind such modulatory effect, additional basic science research is required. In addition, prospective, randomized studies are needed to determine the long-term clinical efficacy of SCS (Fig. 46.3).
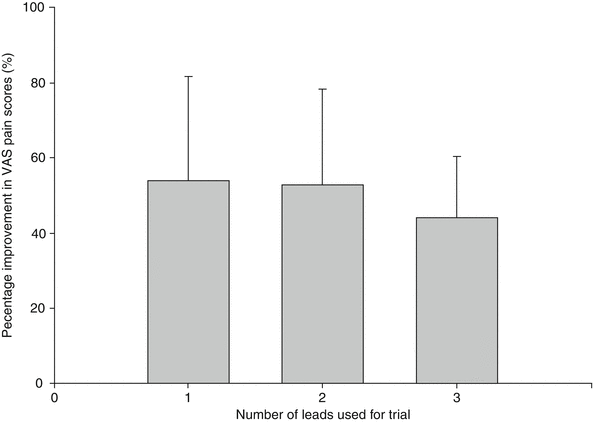

Fig. 46.3
Additional published data on spinal cord stimulation for chronic abdominal pain. It appears that the number of leads used does not influence improvements in pain relief, at least not in below-presented small study. If one, two, or three leads are used in order to provide an optimal trialing, pain relief was comparable at the end of the trial (With permission from Kapural et al. [72])
References
1.
Pare P, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28(10):1726–35; discussion 1710–1.PubMedCrossRef
3.
4.
Giamberardino MA, Vecchiet L. Pathophysiology of visceral pain. Curr Pain Headache Rep. 1997;1(1):23–33.CrossRef
5.
Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 Suppl 1:i2–5.PubMedCentralPubMedCrossRef
6.
Everhart J. Digestive diseases in the United States: epidemiology and impact, P.H.S. US Department of Health and Human Services, National Institute of Diabetes and Digestive and Kidney Diseases, NIH publication No. 94–1447. p. 3–53.
7.
Graney DO. General considerations of abdominal pain. In: Loesser JD, editor. Bonica’s management of pain. Philadelphia: Lippincott, Williams & Williams; 2001. p. 1235–68.
8.
9.
Camilleri M, Ford MJ. Functional gastrointestinal disease and the autonomic nervous system: a way ahead? Gastroenterology. 1994;106(4):1114–8.PubMed
10.
Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 1990;99(6):1688–704.PubMed
11.
12.
13.
14.
Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13(9):1313–32.PubMedCentralPubMedCrossRef
15.
Gebhart GF. Visceral pain-peripheral sensitisation. Gut. 2000;47 Suppl 4:iv54–5; discussion iv58.PubMedCentralPubMed
16.
Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G834–8.PubMed
17.
18.
Kasparov SA. Viscerosomatic convergence on the lumbar interneurons of the dorsal horn of the spinal cord in cats and rats. Neirofiziologiia. 1992;24(1):3–11.PubMed
19.
20.
21.
22.