Fig. 18.1
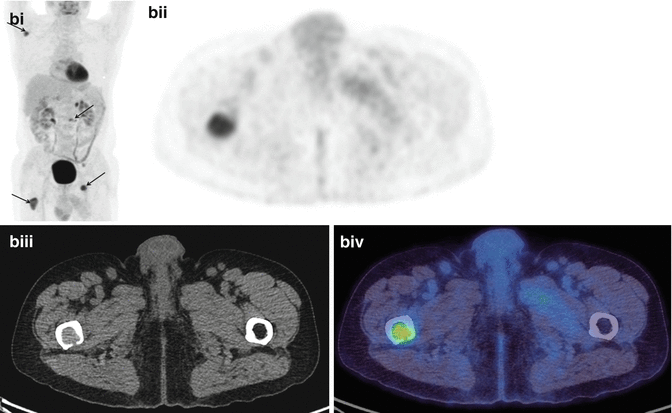
A 67-year-old man had a right upper lobe NSCLC resected. (a) FDG-PET/CT scan (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the thorax demonstrating an active right upper lobe carcinoma with ipsilateral hilar nodal involvement. One year later he complained of bone pain and a follow-up FDG-PET/CT scan (b) (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the right femur showed bone (right femur, left pelvis, right scapula, L2; arrows) and left adrenal gland metastases
FDG-PET has also shown promise in early assessment of targeted therapies [66, 67]. Post-therapy residual FDG uptake in the tumour is a poor prognostic marker [68]. Irrespective of the cell type or neoadjuvant treatment, regression of baseline SUVmax by 80 % is a predictive marker of complete pathological response and with a sensitivity, specificity and accuracy of 90 %, 100 % and 96 % [69].
18.4 Lymphoma
Lymphomas are the most common and most curable form of primary haematological cancer. Surgery has a limited role in management of these patients and the mainstay of treatment is chemotherapy and radiotherapy. With overall survival rates as high as 90 %, early detection and accurate staging are crucial for appropriate treatment planning, and FDG-PET/CT is recommended for initial staging, assessing response to therapy and post-therapy staging of patients with Hodgkin’s disease and high-grade non-Hodgkin lymphomas [70, 71] (Fig. 18.2).
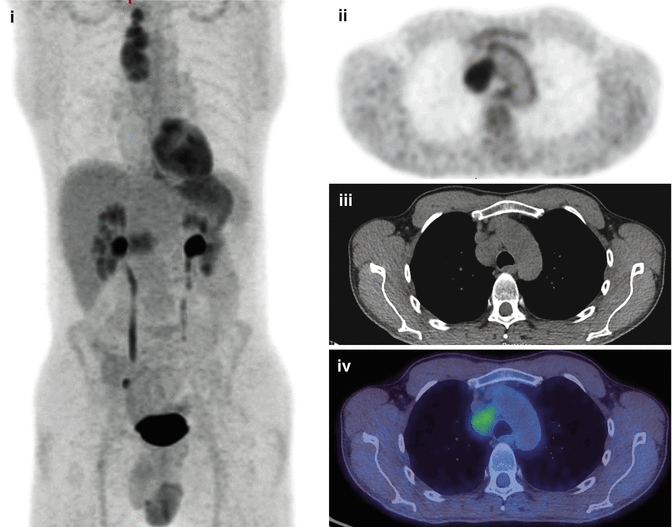

Fig. 18.2
A 27-year-old male with a new diagnosis of Hodgkin’s lymphoma showing FDG-avid nodal disease in the mediastinum with no evidence of extranodal disease. FDG-PET/CT scan (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the mediastinum
Overall sensitivity and specificity for staging and restaging of Hodgkin’s lymphoma are 86 % and 96 %, respectively, which is significantly higher than that by conventional CT imaging (81 % and 41 %, respectively) [72]. Nodal and organ involvement can be predicted with a sensitivity and specificity of 94 % and 100 % with FDG-PET compared to 88 % and 100 % with CT, respectively [73]. FDG-PET/CT is routinely used in diffuse large B-cell lymphoma staging and helps in detecting nodal and extranodal disease sites such as the skeleton, liver and lung [74–76]. In a retrospective review conducted on 130 diffuse large B-cell lymphoma (DLBCL) patients, compared to bone marrow trephine biopsy, FDG-PET/CT was found to have higher overall accuracy in detecting bone and bone marrow (B/BM) involvement by lymphoma, with a sensitivity and specificity of 40 % and 100 % and 94 % and 100 %, respectively. The negative and positive predictive values were 98 % and 100 %, respectively [74]. Low-grade lymphomas, e.g. MALT (mucosa-associated lymphoid tissue) and low-grade follicular lymphoma, are not highly FDG avid and may show higher false-negative rates than in high-grade lymphomas [77, 78].
FDG-PET is useful in differentiating residual tumour from posttreatment fibrosis within residual radiographic masses [79]. Such lesions are seen in up to 50 % of non-Hodgkin’s lymphoma and 66 % of Hodgkin’s disease, and of these only up to 25 % of non-Hodgkin’s lymphoma and 30 % of Hodgkin’s disease patients eventually relapse [80, 81]. A prospective study of 58 patients of which 43 were Hodgkin’s disease with posttreatment residual disease monitored using FDG-PET showed that SUV ≤3 predicts lack of recurrence (P = 0.004) and longer progression-free survival (PFS) (P < 0.00001) [82]. Studies have shown that response assessed on mid-treatment FDG-PET is a prognostic indicator for disease-free and overall survival in HL and aggressive NHL, as early as after one to three cycles of chemotherapy. Residual post-therapy disease is seen up to 85 % of cases of HL and up to 40 % of the cases of NHL. Early interim imaging after 2–4 cycles using FDG-PET/CT is found to correlate well with event-free survival in HL and high-grade NHL [83, 84]. FDG-PET imaging is used to tailor therapies by categorising patients with a worse prognosis into subgroups who will benefit from different types of treatment, such as additional radiotherapy to areas of bulky disease or myelo-ablative chemotherapy followed by stem cell transplantation [79, 85, 86].
18.5 Breast Cancer
Breast cancer is the commonest malignancy in females and is the second leading cause of death. The 5-year survival for localised cancers is approximately 96 % which decreases significantly with regional and distant metastases. FDG-PET is mostly used in the evaluation of recurrent or advanced breast cancer. Other potential clinical applications include staging of axillary lymph nodes and monitoring of response to chemotherapy [87, 88].
Studies have shown no proven role in detecting small and non-invasive primary carcinomas; the overall sensitivity is shown to be only 68 % for tumours of size <2 cm [88, 89]. Dense glandular tissue has relatively high uptake of FDG when compared to adipose tissue within the breast, and hence the tumour contrast is less reliable in dense breast parenchyma [90, 91]. Clinical utility has been shown in the detection of primary invasive breast cancers, mainly infiltrating ductal carcinomas, and in the detection of internal mammary and mediastinal metastases [92]. Further studies reported that the sensitivity of FDG-PET/CT for primary lesions <5 mm was 53 % and 92 % for lesions >20 mm [92]. In large primary cancers with a mean tumour size of 4.3 ± 1.4 cm, the reported sensitivity and specificity of FDG-PET/CT was 77 % and 80 %, respectively [87]. Nodal status is a prognostic and predictive marker of survival in patients with breast cancer. Many clinical studies have evaluated the accuracy of PET imaging in axillary nodal staging of operable breast cancer [93–95]. A systematic review and meta-analysis in 2,460 breast cancer patients revealed that FDG-PET has a wide range of sensitivity ranging from 20 to 100 % and specificity ranging from 65 to 100 % for detecting axillary node metastases [96]. Variable sensitivity (79–94 %) and specificity (86–92 %), and hence insufficient predictive accuracy for axillary nodal staging, preclude the use of FDG-PET as a modality for routine use [97].
For detection of relapse, FDG-PET/CT is found to be more sensitive than the serum tumour marker CA 15-3. It has shown high overall sensitivity, specificity and accuracy for the detection of locoregional recurrence (89 %, 84 % and 87 %, respectively) and distant metastases (100 %, 97 % and 98 %, respectively) [98] (Fig. 18.3). Despite limitations in identifying micrometastases, FDG-PET has an important role in the assessment of breast cancer response to chemotherapy as well as other treatments such as hormonal therapy and radiation [99, 100]. FDG-PET has been used to evaluate response to chemotherapy, and studies have demonstrated that responding tumours have a 40 % or more regression of SUVmax, whereas nonresponding tumours may show increased, stable or a slight decline of SUVmax which is not more than 24 %. The prognosis also correlates in a similar pattern, and patients who show more than a 40 % decrease in SUVmax are found to have a better prognosis [24, 87, 98, 101]. Reduction in FDG activity in cases of bone metastases with associated sclerosis in the CT component of FDG-PET/CT is a sign of response and bone healing [102, 103].




Fig. 18.3
A 39-year-old female with a 3-year history of breast cancer and known relapse referred for restaging. The FDG-PET/CT scan (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the right breast (a) and right pelvis (b) shows right breast recurrence with several bone metastases including the sternum, pelvis and femora. There is some physiological brown fat activity but also some right axillary nodal recurrence (ai)
Another important PET tracer is 18 F-fluoroestradiol (FES) which is found to correlate with oestrogen receptor (ER) expression has been used to localise ER-expressing tumours. It has been shown to predict response to salvage hormonal treatment in heavily pretreated metastatic breast cancer patients [104]. In a study of 47 patients with recurrent or metastatic breast cancer with ER-positive tumours, the treatment selection-based quantitative FES-PET showed that the response rate increased from 23 to 34 % overall and from 29 to 46 % in patients lacking HER2/neu overexpression suggesting that quantitative FES-PET can be used for guiding treatment selection [105].
18.6 Malignant Melanoma
Malignant melanomas are known to metastasise extensively anywhere in the body including unusual sites such as meninges, the gastrointestinal tract and myocardium [106, 107]. As these tumours show high FDG uptake, FDG-PET is a sensitive modality for staging patients with high-risk melanomas [108–110]. The reported sensitivity of FDG-PET for detecting visceral and abdominal nodal metastases is as high as 100 % with an accuracy of 100 % for superficial lymph node metastases. However, it has a lower sensitivity for pulmonary metastases, and the CT component of a combined FDG-PET/CT scan allows better evaluation of small pulmonary metastases [111]. A known limitation is in detecting metastases in the brain where normal parenchyma has a high background activity. As compared to CT imaging alone and PET alone, the overall diagnostic accuracy for M staging is significantly higher for FDG-PET/CT (84 %, 93 % vs. 98 %, respectively) (Fig. 18.4). Similarly for N staging, the diagnostic accuracy of CT alone is reported to be 86 %, whereas for FDG-PET/CT, it is 98 %. Baseline PET/CT staging in stage I–IV patients may change treatment in 48.4 % of patients [108, 111–113].


Fig. 18.4
A 30-year-old male with a previous history of a back melanoma referred for routine follow-up imaging. The FDG-PET/CT scan, (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the thorax, shows a small recurrent skin lesion on the back
18.7 Head and Neck Cancer
PET imaging using FDG has a significant role in both newly diagnosed as well as treated patients with head and neck cancers [114]. Compared to CT imaging alone, FDG-PET/CT changes the initial clinical stage and TNM category of tumours in 14–57 % of patients with a diagnostic accuracy of approximately 90 % compared with 86 % for conventional CT [115–118]. For lymph node metastases, the per-patient sensitivity and specificity of FDG-PET/CT are reported as 94 % and 84 %, respectively, in comparison to 78 and 84 % for CT alone [119]. FDG-PET/CT imaging has been reported to change the initial management in 18–37 % of patients. It may detect synchronous lesions in 8.1 %, the site of unknown primaries in 73 % and distant metastatic lesions in 15.4 % of patients with head and neck cancer [117, 120]. It also has implications in radiotherapy planning and may influence changes due to the gross tumour volume (GTV) planned by FDG-PET/CT in 57 % of patients leading to a change in the planning radiotherapy field in approximately 29 % of patients [121, 122]. The sensitivity, specificity and accuracy of FDG-PET/CT in restaging patients with head and neck cancer have been reported to be 88 %, 78 % and 86 %, respectively [123].
The pretreatment SUV of the tumour on FDG-PET may have prognostic potential. Machtay et al. found that patients whose lesions showed maximum SUV <9 had 72 % disease-free survival compared to 37 % in patients with lesions having SUV >9. FDG-PET is found to have a very high negative predictive value for the detection of post-therapy recurrence [124]. However, in this setting FDG-PET has a low predictive value in detecting occult nodal metastases with a low positive predictive value due to false-positive lesions related to infective and inflammatory lesions or posttreatment effects. This limitation is partially overcome by FDG-PET/CT, as the posttreatment changes, inflammatory lesions and physiological structures such as brown fat are easily distinguishable from abnormalities on FDG-PET/CT images, yet are more challenging to differentiate by PET or CT alone [125–127]. For the detection of primary site residual/recurrent disease and nodal recurrence, FDG-PET/CT is found to have higher sensitivity (88–100 %) and specificity (75–100 %) than CT alone (sensitivity, 38–90 %; specificity, 38–85 %) [128].
18.8 Thyroid Cancer
Approximately 90 % of thyroid malignancies are papillary and follicular carcinomas with well-differentiated morphology [129]. As the tumours become dedifferentiated, they tend to lose sodium iodide symporter expression; this causes inability of the cells to concentrate radioiodine. Similar features are seen in recurrent thyroid cancers and metastatic tumours [130]. In a multicentre trial in patients with increased levels of thyroglobulin and negative whole-body 131-iodine scans, FDG-PET/CT has shown a sensitivity of 85 % and it changed clinical management in 23–51 % of patients [131]. In iodine-refractory thyroid cancer, FDG-PET is found to be a predictive marker for tumour aggressiveness [132–135] (Fig. 18.5).
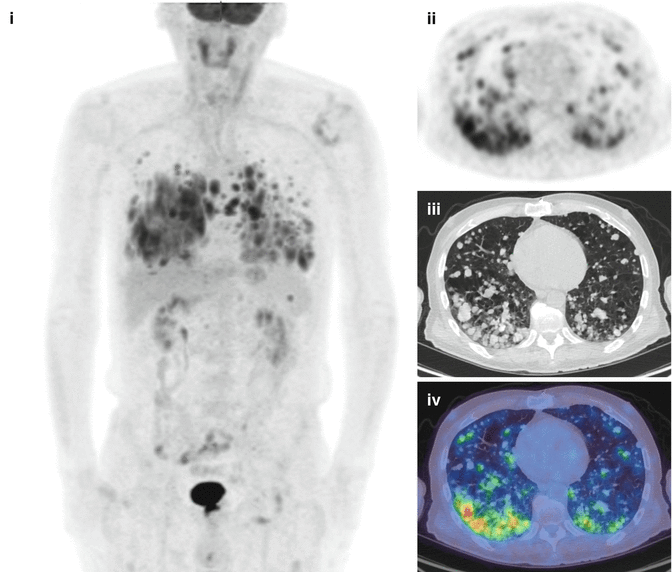

Fig. 18.5
A 62-year-old male with undifferentiated thyroid cancer demonstrating many FDG-avid pulmonary metastases. 131-iodine scans were negative. (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the thorax
18.9 Oesophageal Cancer
FDG-PET/CT is recommended for initial M staging of oesophageal cancer and detects unexpected metastatic foci which are present in up to 30 % of patients [136–138]. Synchronous primary oesophageal tumours are detected in 5.5 % of patients which otherwise were not identified on conventional imaging [139–141]. It results in downstaging of disease in 5–7 % and upstaging in 15–20 %. For the detection of distant metastases, FDG-PET/CT has an overall sensitivity, specificity and accuracy of 43–78 %, 93–99 % and 62–86 %, respectively, which is better than CT and endoscopic ultrasound (EUS) [142, 143] (Fig. 18.6). FDG-PET has a diagnostic accuracy of 85 % in assessment of therapeutic response to neoadjuvant chemotherapy and is found to be an important prognostic factor [144, 145]. Thirty percent or more regression in the SUVmax is found to be a predictive marker of response to the chemotherapy, and 50 % or more decrease in SUVmax correlates with long-term disease-free survival and overall survival [146, 147]. For inoperable cases FDG-PET has been found to help in radiotherapy planning and has shown 56 % modification of gross tumour volume leading to alteration of treating volume in 53 % patients [148]. FDG-PET is highly sensitive in detecting regional and distant recurrent lesions with a reported sensitivity, specificity and accuracy of 94 %, 82 % and 87 %, respectively, in comparison to 81 %, 82 % and 81 % for conventional imaging [149].




Fig. 18.6
A 74-year-old male with carcinoma of the gastro-oesophageal junction. FDG-PET/CT shows increased activity in the primary tumour (black arrow) (a) as well as an involved coeliac lymph node (red arrow) (b). (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the primary tumour (a) and upper abdomen (b)
18.10 Colorectal Cancer
FDG-PET/CT has a role in therapy assessment, imaging of recurrent disease, localisation of recurrent disease in unexplained raised serum carcinoembryonic antigen (CEA) levels and staging before surgical resection of local recurrence and distant metastatic disease in colorectal malignancy [150–152]. Routine use of FDG-PET/CT in the initial staging is not usually justified and is reserved as a problem solving tool for evaluation of high-risk patients such as CEA levels >10 ng/ml with locally advanced disease or equivocal findings on conventional imaging [153–155]. FDG-PET/CT not only detects unsuspected metastatic disease but also helps determine the nature of indeterminate lesions, and in turn it is found to change management in 18–24 % of these high-risk category patients [156–158]. In patients with raised CEA and negative or equivocal conventional imaging, FDG-PET/CT has shown a sensitivity of 88 %, specificity of 94 % and accuracy of 92 % for the detection of extrahepatic intra-abdominal colorectal recurrence and 95 %, 100 % and 99 %, respectively, for extra-abdominal and/or hepatic recurrences (Fig. 18.7). The overall sensitivity, specificity and accuracy for diagnosing recurrent colorectal disease are 89 %, 92 % and 90 %, respectively [159].


Fig. 18.7
A 58-year-old male with a history of rectal cancer with a rising CEA during follow-up. FDG-PET/CT scan shows active peritoneal metastases. (i) MIP, (ii) PET, (iii) CT and (iv) fused PET/CT axial images through the upper abdomen. A left ureteric stent is present as the patient had presented with hydronephrosis
18.11 Gynaecological Cancers
18.11.1 Cervical Cancer
In advanced cervical cancer, FDG-PET/CT has an established role in preoperative staging and post-therapy restaging [162, 163]. For the detection of para-aortic nodal disease in advanced cervical cancer with negative conventional CT imaging, FDG-PET has shown a sensitivity, specificity and accuracy of 86 %, 94 % and 92 %, respectively. Preoperative FDG-PET imaging has been found to influence patient management in 18 % of patients [164]. For the detection of recurrent disease, FDG-PET imaging has an overall sensitivity and specificity of 86–94 % and 76–100 %, respectively. Post-therapy absence of FDG uptake is found to correlate with 2-year progression-free survival (86 % compared to 40 % in patients who showed persistent residual FDG uptake) [165, 166].
18.11.2 Ovarian Cancer
FDG-PET/CT is not routinely used for the detection of ovarian cancer [167, 168]. Studies have reported a sensitivity of 87 % and specificity of 100 % for differentiating benign from malignant ovarian cancer. However, it has a low diagnostic value in differentiating between borderline and benign tumours. Lesions up to 5 mm can be diagnosed [169]. Though it has a high specificity, its sensitivity is much lower than that of sonography [170, 171]. FDG-PET/CT is useful in evaluating distant metastatic disease. Pretreatment staging accuracy of FDG-PET/CT is found to be 69–75 %, compared with 53–55 % with CT alone [172, 173]. High sensitivity has been reported for identifying peritoneal deposits larger than 1 cm and lymph nodes larger than 7 mm [174]. FDG-PET/CT identifies patients who are not suitable candidates for optimal debulking and would need preoperative chemotherapy. Early diagnosis of recurrence and exact anatomic localisation of metastatic disease are crucial for determination of the best treatment strategy [175–177]. There are limited data available regarding the role of FDG-PET or FDG-PET/CT to monitor therapy response in ovarian cancer. In one study sequential FDG-PET/CT used to predict patient outcome showed a significant correlation between changes in tumour FDG uptake after the first and third cycles of neoadjuvant chemotherapy in advanced-stage (International Federation of Gynecology and Obstetrics stages IIIC and IV) ovarian cancer, but not with conventional clinical or CA-125 response criteria [178, 179]. High rates of complete tumour resection were achieved in cases which showed more than 20 % regression in SUV from baseline after the first cycle and 50 % after the third cycle than in nonresponders. Also the rate of macroscopic tumour-free surgical resection was 33 % in metabolic responders vs. only 13 % in nonresponders [179]. In recurrent ovarian cancers with raised serum CA-125 and negative conventional imaging, FDG-PET/CT has a reported sensitivity and PPV of 83.3 % and 93.8 %, respectively, for the detection of recurrent disease at least 1 cm in size [180].
18.12 Pitfalls
Important pitfalls of FDG-PET/CT imaging can be seen during interpretation of scans. The factors which have been proved to cause variation in the detection of tumour include tumour size, tumour metabolic activity, background activity of surrounding tissue and serum glucose levels. False-negatives are commonly found in smaller lesions (<7 mm), tumours having low metabolic rate, interference from cytostatic treatments which cause a decrease in the tumour FDG uptake in the presence of viable tumour and suboptimal patient preparation, especially in patients with glucose intolerance or diabetes leading to a decrease in FDG uptake in tumours owing to competitive inhibition with glucose. Other known limitations are high background physiological FDG activity such as in the brain parenchyma and the genitourinary tract which may mask malignant pathologies and hence reduce lesion detection. Benign pathologies such as infections, inflammatory processes and postoperative and post-radiation changes may lead to increased FDG uptake and false-positive results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree