Clinical features
Frequency %
Mediators
Flushing
85–90
Kallikreine, histamine, 5-HT, prostaglandins, substance P
• Pink/red/purple
• Face, neck, upper chest
• Limited duration
Diarrhea
70
Gastrin, 5-HT, prostaglandins, histamine, vasoactive intestinal peptide
• Secretory (watery)
Abdominal pain
35
Obstruction, mesenteric fibrosis, hepatomegaly
• Variable
• Colic or ischemic type
Heart failure
5-HT, substance P
• Right
30
• Left
10
Telangiectasia
25
Unknown
• Purplish vascular lesions
• Nose, upper lip, malar areas
Bronchospasm
10–20
Histamine, 5-HT
• Wheezing
• Dyspnea
Pellagra
5
Niacin deficiency
• Dermatitis
• Glossitis
• Stomatitis
• Mental confusion
The development of carcinoid syndrome is due to liver metastases, which secrete vasoactive mediators directly into the systemic circulation, bypassing the liver. However, it may also occur in the absence of liver metastases, if there is direct retroperitoneal involvement, primary ovarian, or bronchial neuroendocrine tumor. At the time of diagnosis, 20–30 % presents with an acute carcinoid syndrome [3, 5, 6, 8]. Carcinoid syndrome associated with bronchial NETs is often atypical and presents as episodic flushing, diaphoresis, tremor, periorbital edema, lacrimation, salivation, and edema [5, 6]. Carcinoid crisis is a life-threatening expression of the carcinoid syndrome that arises when overwhelming amounts of humoral factors are released into the systemic circulation. It can occur spontaneously or iatrogenic. A list of provoking factors is given in Table 15.2 [5, 6, 9, 10].
Strong evidence | Mild evidence (reported in case reports and patients’ guidelines) |
|---|---|
Palpation | Alimentary triggers |
• Bedside | • Alcohol |
• Intraoperative | • Amine-containing food (e.g., caffeine drinks, chocolate, wine, etc.) |
• Abdominal ultrasound | • Serotonin containing food (e.g., bananas, pineapples, tomatoes, etc.) |
Procedures | Stress |
• Chemotherapy | • Emotional |
• Hepatic artery ligation | • Physical |
• Embolization | |
• Radio nucleotide therapy | |
• Fine needle biopsy | |
• Induction of anesthesia | |
Drugs | Drugs |
• Beta-adrenergic agonists | • SSRIs |
• (Nor)epinephrine |
It is characterized by profound flushing, hemodynamic instability (tachycardia and hypotension), arrhythmias, bronchoconstriction, reversible right ventricular dysfunction, and altered mental state [5, 9, 12].
Pathophysiology
As many as 40 secretory products have been identified in various carcinoid tumors (Table 15.1). They can be divided into three main groups: the amines, the polypeptides, and the prostaglandins.
The three most important substances are serotonin, histamine, and the kinin peptides (tachykinins and bradykinins), which can induce the carcinoid syndrome. Other less important mediators are prostaglandines, vasoactive intestinal peptide, adrenocorticotrophic hormone, and motilin [5].
These vasoactive substances are secreted into the portal circulation and metabolized within the liver. As a result of this first-pass effect, most NETs do not cause the features of a carcinoid syndrome. The syndrome occurs when the venous drainage empties directly into the systemic circulation, which is typically seen in patients where hepatic metastases are present, when there is retroperitoneal involvement or where the primary tumor is situated outside the gastrointestinal tract (e.g., bronchial, ovarian, or testicular neuroendocrine tumors) and releases hormones directly in the systemic circulation [6].
Most of the tumors of the jejunum, ileum, proximal colon, and appendix (>70 %) and some NETs of the stomach and respiratory tract (10–35 %) secrete serotonin [5-hydroxytryptamine (5-HT)], a metabolic derivate from tryptophan (Fig. 15.1) [8]. Serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system. However, most of the serotonin (>90 %) is located in the enterochromaffin cells in the gastrointestinal tract. There it plays an important role in controlling the gastrointestinal motility, sensitivity, and secretion. Furthermore, when it is stored in platelets, it plays an important role in the hemostasis. In the brain, it has various functions, controlling cognitive functions, mood, appetite, and sleep. Adrenergic stimulation is responsible for the release of serotonin into the circulation [13, 14].
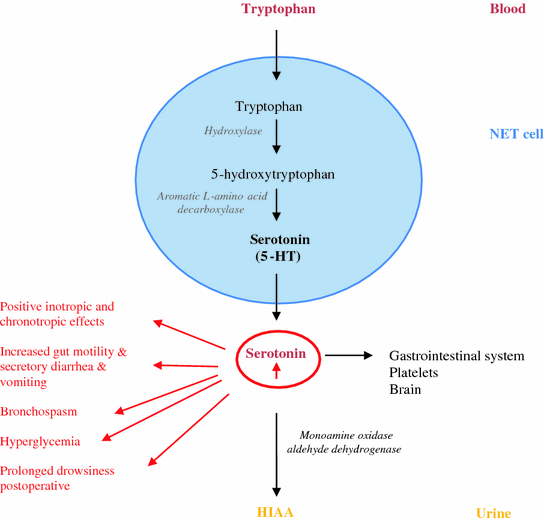

Fig. 15.1
Pathways of serotonin metabolism (5-HT: 5-hydroxytryptamin; 5-HIAA: 5-hydroxyindoleacetic acid)
In patients without the carcinoid syndrome, approximately 1 % of dietary tryptophan is converted to serotonin. This value may increase to 70 % in patients with carcinoid syndrome [5, 6]. Tryptophan is used for the synthesis of serotonin, proteins, and nicotinic acid. As a result of tryptophan deficiency, decreased protein synthesis, hypoalbuminemia, nicotinic acid deficiency, and pellagra can develop. Once serotonin is secreted, it is oxidized and dehydrogenated by aldehyde dehydrogenase and monoamine oxidase to 5-hydroxyindoleacetic acid (5-HIAA), which is ultimately excreted by the kidney. Elevated levels of 5-HIAA are monitored in a 24-h urine sample as a marker of excess serotonin production and represents the presence of a NET. The elevated levels of serotonin can cause positive inotropic and chronotropic responses, increased gut motility, secretory diarrhea, vomiting, bronchospasm, hyperglycemia, and prolonged drowsiness following anesthesia (Fig. 15.1). Histamine release is mostly seen in patients with foregut NET and is probably responsible for the bronchoconstriction and flushing. This theory is however controversial. At least, kinins (e.g., bradykinins and tachykins) can also be released. Peripheral effects of bradykinin, a kinin produced by kallikrein, include hypotension, vasodilatation with flushing, increased capillary permeability, and bronchoconstriction. Tachykinins (e.g., substance P, …) are involved in the development of carcinoid heart syndrome or episodic flushing.
Diagnosis
Diagnosis is often delayed due to the atypical presentation of NETs [1, 6]. Histologic diagnosis requires expertise and includes the use of immunohistochemistry for chromogranine and synaptophysin and also in some tumors for other peptides and the determination of the proliferation index (Ki67) and of the mitotic index [15]. Moreover, imaging (radiology, endoscopy, and nuclear imaging) plays a crucial role in the diagnosis and staging of NETs. Serum markers also contribute to diagnosis: Serum chromogranin A (CgA) is usually found in high concentrations regardless of hormone-related symptoms. However, CgA can be elevated in patients with atrophic gastritis and anacidity, in patients taking proton pomp inhibitors, and in patients with renal insufficiency [8, 15]. In case of a serotonin-producing NET, the work-up includes a 24 h urine sample for 5-HIAA [1]. Urinary 5-HIAA appears to be a good biological marker for the assessment of carcinoid symptoms by a NET and its association with perioperative morbidity. Overall, high preoperative urinary 5-HIAA measurement is a risk factor for perioperative complications, including death. Serial measurements of 5-HIAA are used to monitor disease progression in these patients, although today the serial measurement of serum chromogranin A is more often done [10].
Normal levels of 24 h urinary 5-HIAA are less than 10 mg. Levels greater than 25 mg per 24 h have been considered to be diagnostic for carcinoid tumors. During the collection of the 24 h urine sample, patients should avoid serotonin-rich food (e.g., bananas, pineapples, tomatoes, kiwis, eggplant, plums, plantain, and walnuts) and drugs which will affect urinary excretion of 5-HIAA (e.g., naproxen, paracetamol, etc.) [1, 6, 15]. However, up to 20 % of patients with carcinoid symptoms have normal 5-HIAA levels. Additional screening for insulin, C-peptide, gastrin, VIP, pancreatic polypeptide, glucagon, and calcitonin should depend on the clinical symptoms, histological features, and functional status of the tumor.
Management of Carcinoid Syndrome
The primary treatment goal, and currently the only possible cure, for patients with gastrointestinal NETs, is curative surgery. Curative surgery is, however, often not possible, since most patients present with metastases at the moment of diagnosis. As curative treatment is not possible, many patients require chronic medical management to relieve symptoms and to suppress tumor growth. Symptom control can be done by somatostatine analogs (SSA). At this moment, three SSAs are available: octreotide, lanreotide, and pasireotide. SSAs act by binding to somatostatin receptors, which are expressed on the majority of NETs. There are five receptors (SST1–SST5), linked with several intracellular systems: reduction in calcium inflow, inhibition of adenylyl cyclase, and in some tissues stimulation of tyrosine phosphatase activity. Activation of SST1 and SST2 is associated with antimitotic activity of SSA. Activation of SST5 is responsible for the reduction in calcium inflow, which is correlated with inhibition of cell proliferation. The SST3 receptor mediates apoptosis. Another working mechanism of SSA is inhibition of growth factor and angiogenesis, as well as immunomodulatory effects. The SST2 receptor is the most frequent somatostatin receptor on NETs (90 % of serotonin-secreting NET and 80 % on pancreatic NET). Native somatostatine binds to all receptors, but octreotide has high affinity for SST2 and lower affinity for SST3 and SST5 [7, 16–18].
Octreotide was the first approved SSA. It can be administered as a short-acting octeotride that is usually administered three times daily subcutaneously (SC) (Sandostatine® 0.1–0.5 mg) and a slow-releasing form of octreotide injected intramuscular monthly (Sandostatine LAR® 20–30 mg), both controlling symptoms equally. If a patient is changed to the long-acting formulation, the time required to reach steady-state levels makes continuation of subcutaneous octreotide administration necessary for at least 2 more weeks [19]. The antitumor efficacy of SSA appears weak, even if it is used in high dosages. However, disease stabilization occurs in 50–60 %. Recently, the first randomized, double-blind, placebo-controlled, multicenter, phase III study of octreotide LAR in patients with metastastic well-differentiated neuroendocrine midgut tumors was published. This study (PROMID) shows that octreotide LAR inhibits tumor growth in patients with metastatic midgut NETs, without a difference between functioning and non-functioning tumors. The antiproliferative effect was presumably more in patients with low (<10 %) hepatic tumor load or after a resected primary tumor. Although further studies are needed, the number of patients with high tumor load was low [5, 6, 20, 21]. Another SSA is lanreotide (BIM-23014, Somatuline Depot®), a long-acting formulation, which is available in monthly injections at doses ranging from 60–120 mg SC every 4 weeks. Octreotide and lanreotide have similar clinical efficacy and tolerability for the treatment of carcinoid syndrome [15]. Patients receiving octreotide or lanreotide may experience an ‘escape from response’ 6–18 months after initiation due to progression of the disease or tachyphylaxis. Pasireotide (SOM-230), a newer SSA, with high binding affinity for SST1, SST2, SST3, and SST5, is under development in patients with symptomatic NETs. The most common side effects of SSAs are abdominal bloating and discomfort; mostly, they are mild and disappear spontaneously within the first week. Diminished gallbladder contractility can develop, leading to gallstones or sludge, but only a small proportion of patients develop symptoms. According the ENET guidelines, preventive cholecystectomy is not required [15].
In NET patients with carcinoid symptoms, with progressive disease under and/or symptoms not controlled by SSA, other antitumoral therapies have to be considered. Interferon-α therapy (IFN, Intron®) is generally recommended as a second-line approach in patients with functional and non-functioning NETs and low proliferation index, to control refractory symptoms. It is used at doses titrated by side effects and leukocyte count. The effect of IFN on symptom control is similar to that of somatostatin analogs; however, they do not act as rapidly, and it has a less favorable safety profile. NET patients, with symptoms not under control by SSA and with a slowly progressive tumor with a low proliferation index and a negative somatostatin receptor scintigraphy, are potential candidates for IFN. Liver-targeted therapies to debulk tumors and improve symptoms, such as surgical resection, transarterial embolization (TAE) with or without selective artery infusion of chemotherapy (TACE), and radiofrequency ablation (RFA), play an important role in the management of patients with advanced NET with liver metastases. It can control tumor growth and the carcinoid and/or tumor-related symptoms when medical therapy failed. However, if palliative surgery is performed, it is often recommended to resect at least 90 % of the tumor. It is still unclear which percentage of the tumor bulk has to be resected to achieve improvement in symptom control and outcome. More prospective clinical trials are clearly needed [15, 21, 23]. Everolimus and sunitinib are recommended in patients with good or moderately differentiated progressive pancreatic NETs. Chemotherapy is recommended in progressive pancreatic NETs and also in the poorly differentiated NETs of any site. Peptide receptor radionucleotide therapy (PRRT) with 90Y- and/or Lu-DOTATOC or –DOTATATE may be used to treat metastatic somatostatin receptor-expressing NET [15]. Patients with end-stage liver disease and uncontrollable symptoms unresponsive to any other therapy have been considered for orthotopic liver transplantation. Although the shortage of organs in combination with low survival data suggests that liver transplantation should only be considered in exceptional circumstances and further studies are needed [8].
Antidiarrheal agents such as loperamide, diphenoxylate, or atropine can be used for refractory symptoms. For more severe diarrhea, opiates (tincture of opium) may be prescribed. Several studies propose serotonin receptor antagonists; however, the benefit is small. Patients with bile acid malabsorption, occurring after resection of the small bowel, can be treated with cholestyramine or colestipol [ 24, 25]. Fluid and electrolyte abnormalities should be corrected.
Preoperative, Intraoperative, and Postoperative Management to Prevent a Carcinoid Crisis
Preoperative management should include a detailed medical history and clinical examination to uncover the presence and the severity of the carcinoid syndrome. Furthermore, presenting triggering factors should be determined. Routine investigation should include a complete laboratory screening with blood count, glucose, liver and renal function and electrolyte screening, as well as a urinary 5-HIAA determination, electrocardiography, and echocardiography. The tumor staging and classification should be complete.
Preoperative management is focused on relieving symptoms and prevention of a potential carcinoid crisis. This is achieved by antagonizing the mediators of carcinoid disease and blocking their release. The effect of histamine is antagonized by H1-blocker (e.g., cetirizine) and H2-blocker (e.g., ranitidine). Bradykinin can be blocked by aprotinin, a kallikrein inhibitor, which is useful for the treatment of flushing and perioperative hypotension, refractory to octreotide. There are, however, conflicting case reports for the treatment of perioperative hypotension [6]. Several other agents have been used in perioperative management, including steroids for bronchospasm, methylsergide, and cyproheptadine for gastrointestinal manifestations and ketanserin, which blocks the effect of serotonin mediated at the 5-HT2 receptor, namely vasoconstriction, bronchoconstriction, and platelet aggregation, and can be used to treat hypertension [5, 6]. Anxiolytic premedication (e.g., alprazolam) that does not have histamine-releasing properties is recommended to reduce the release of catecholamine as a result of preoperative stress [5, 6]. The prevention of a carcinoid crisis should be performed prior to surgery and locoregional interventions, using SC or IV SSA. Various regimes for dosing SSA preoperatively have been reported; however, the optimal dose had not been studied systematically. Subcutaneously, it is given at a dose of 0.5 mg three times daily, 2 weeks prior to surgery, and given for 1 week after surgery. If it is to be discontinued postoperatively, it should be reduced slowly over the first postoperative week. Intravenously, it is used at a dose of 50–100 μg per hour, initiated 24 h before, and given for 24–48 h after the surgical intervention. Furthermore, the patient’s maintenance medications should be continued [8, 15]. Octreotide has been successfully used during orthotopic liver transplant for NET at a dose of 0.05 mg IV injection prior to incision, followed by a continuous infusion of 0.05 mg per hour, and additional 0.05 mg boluses to treat episodes of hemodynamic instability. Following this management, there were still episodes of hypotension, related to surgical manipulation of the liver, but never life-threatening [9].
Intraoperative invasive monitoring starting before induction, with an arterial and central venous pressure (CVP) catheter to detect hypovolemia and hypotension, airway pressure monitoring to detect the onset of bronchospasm, temperature monitoring, to detect hypothermia and monitoring of the urinary output, is recommended. In patients with cardiac dysfunction, monitoring of left ventricular function by a Swan-Ganz catheter can be useful. The ideal induction agent is propofol; however, hypotension should be avoided [26]. Etomidate can cause histamine release and may not suppress laryngeal reflexes, but is cardiovascularly more safe [ 27, 28]. Furthermore, only non-depolarizing neuromuscular blocking agents that do not cause histamine release should be used. The best choice is vecuronium or rocuronium [6, 28]. Opioids that do not cause histamine release should be used for analgesia. Intravenous fentanyl has been used with good effect and no adverse events. We suggest to give 0.5 mg octreotide IV before induction to prevent mediator release.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree