Study
Tumor
N
Treatment
Crossover (%)
Prior CT (%)
Prior SSA (%)
Concurrent SSA (%)
ORR (%)
P
PFS (m)
HR
P
OS
HR
P
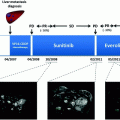
NCT00428597
Pancreatic NETs
171
Sunitinib
69
57
35
27
9.3
<0.007
11.4
0.42
<0.001
30.5 m
0.74
NS
Placebo
61
38
30
0
5.5
25.4 m
RADIANT-3
Pancreatic NETs
410
Everolimus
73
50
50
39
5
NS
11.4
0.34
<0.001
73 % (18 m)
1.05
NS
Placebo
50
50
40
2
5.4
74 % (18 m)
RADIANT-2
Functional NETs
429
Everolimus + octreotide LAR
58
35
80
–
3
NS
16.4
0.77
= 0.026 (one-ided)
71 % (18 m)
1.22
NS
Placebo + octreotide LAR
26
78
–
2
11.3
74 % (18 m)
Bevacizumab
Bevacizumab is a humanized anti-VEGF monoclonal antibody that has demonstrated efficacy in a wide spectrum of solid tumors, including colorectal, breast, renal, and non-small cell lung cancer. A small randomized phase II trial conducted by Yao et al. also suggested this drug could have some activity in GEP-NETs [13]. In this study, 44 patients with metastasic or unresectable carcinoid tumors on stable doses of octreotide were randomly assigned to 18 weeks of treatment with bevacizumab (15 mg/kg intravenously once every 3 weeks) or PEG interferon alfa-2b (0.5 mcg/kg subcutaneously once per week). After the completion of the 18 week therapy or at disease progression (whichever occurred earlier), patients were allowed to receive the combination of the two drugs. The bevacizumab arm showed higher response (18 vs. 0 %) and PFS rates after 18 weeks of treatment (95 vs. 68 %). In addition, a significant decrease in tumor blood flow (BF) as measured by paired functional CT scans was observed among patients treated with bevacizumab but not among those treated with interferon. Regarding toxicity, neutropenia was more frequently observed in the PEG interferon alfa-2b arm (14 vs. 0 %, p = 0.02) and hypertension in the bevacizumab arm (18 vs. 0 %, p = 0.01). Other than that no significant differences were observed among study arms in terms of fatigue, nausea, vomiting, headache, or myalgia. A large phase III study is currently ongoing (SWOG S0518) to try to confirm these promising results (trial estimated completion date: January 2012; expected enrollment: 400 patients). Another randomized run-in study of bevacizumab versus everolimus for 21 days, followed by the combination of both drugs, is currently assessing the role of functional CT scans as surrogate markers for selection of patients likely to benefit from antiangiogenic therapies [14]. Preliminary results of this study, that included 39 patients with low- to intermediate-grade NETs, showed that treatment with bevacizumab significantly decreased tumor BF, and this was further reduced with the addition of everolimus. Moreover, a significant association was observed between objective responses (21 %) and functional CT scan parameters such as higher baseline permeability surface, higher post-treatment mean transit time (MTT), higher percentage decrease in BF, and higher percentage increase in MTT. Confirmatory studies of these provocative findings are warranted. Also suggesting enhanced antitumor effects with combined mTOR and VEGF-targeted therapy are the preliminary results recently reported of a multicenter phase II trial testing the combination of temsirolimus and bevacizumab in patients with progressive well to moderately differentiated PNETs, with an objective response rate (44 %) that well exceeds that expected from single agent therapy. Finally, results from the BETTER trial have been recently reported, which tested the combination of bevacizumab and capecitabine in 49 patients with non-pancreatic NETs of the GI tract (40 from the small intestine, 3 from the cecum, 4 from the rectum, and 2 from the stomach), all with Ki-67 proliferative index <15 % (35 % of 0–2 %) [15]. Tumor control rate was observed in 88 % of patients, including partial responses in 9 (18 %), and the PFS rate at 18 months was 55 %. Grade 3/4 adverse events occurred in 41 patients (84 %), mainly gastrointestinal toxicities (29 %) and hypertension (31 %). Several additional phase II trials are evaluating safety and efficacy of a number of bevacizumab combinations with other cytotoxic drugs [(FOLFOX, XELOX, Temozolomide) or targeted agents (temsirolimus, pertuzumab, sorafenib) (see below)].
Other Angiogenesis Inhibitors
Sorafenib is a small molecule currently approved for the treatment of hepatocellular and renal cell carcinoma, which inhibits tumor-cell proliferation and angiogenesis by inhibiting, among others, the serine–threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3, and platelet-derived growth factor receptor β (PDGFR-β). A phase II trial reported the outcome of 93 patients with advanced PNETs and carcinoid tumors that were treated with sorafenib 400 mg bid [16]. Among evaluable patients, 4 of 41 patients (10 %) in each group achieved a partial response, and 3 and 9 minor responses were observed in patients with PNETs and carcinoid tumors, respectively. Preliminary results (presented at ASCO 2007, but not yet published), showed a 6 month PFS rate of 40 % for carcinoid tumors and 61 % for PNETs. With regard to safety, grade 3–4 adverse events occurred in 43 % of patients, being fatigue (9 %), skin (20 %) and gastrointestinal toxicities (7 %) the most commonly encountered. Although these results were considered to indicate a modest activity of sorafenib in this patient population, and are not substantially different from that observed for some of the recently approved drugs for this indication, further development in prospective randomized trials has not followed. However, different combinations of sorafenib with other biological or cytotoxic agents, such as everolimus or cyclophosphamide, are being explored in non-controlled trials. In addition, combinations with other antiangiogenic agents are also being assessed. Indeed, the Spanish Neuroendocrine Tumor Group (Grupo Español de Tumores Neuroendocrinos or GETNE) has recently reported the early results of a phase II study evaluating the combination of sorafenib (200 mg bid, days 1–5 of each week) and bevacizumab (5 mg/kg once every 2 weeks), based on the hypothesis of a potential synergistic effect derived from the complementary VEGF “vertical” signaling inhibition [17]. Forty-four patients with advanced G1–2 NETs were enrolled in this trial (n = 31 carcinoid tumors and 13 with PNETs). Most common grade 3–4 toxicities were hand-foot syndrome (23 %), asthenia (16 %), hypertension (9 %), and mucositis (7 %). Seven patients prematurely ended the study treatment due to adverse events. The overall response rate by RECIST criteria was 9.8 %. Disease control rate (95 %) and median PFS (12.4 months) are encouraging, although further follow-up is required for mature data. Other ongoing trials include combinations of sorafenib with conventional cytotoxic agents (cyclophosphamide-NCT00605566) or other targeted agents such as everolimus.
Pazopanib is a second-generation orally available multitargeted tyrosine kinase inhibitor against vascular endothelial growth factor receptor-1, 2, and 3, platelet-derived growth factor receptor-α, platelet-derived growth factor receptor-β, and c-kit. Preclinical evaluation has demonstrated significant antiangiogenic properties and antitumor activity in a variety of tumor types, and phase I clinical trials have revealed manageable toxicities as well as activity in renal cancer and several other malignancies. A prospective phase II study evaluated the combination of pazopanib and depot octreotide in 2 cohorts of patients with PNETs (n = 30) and carcinoid tumors (n = 22) following a two-stage design, with early stopping rules if no RECIST-defined response was observed among the first 20 evaluable patients enrolled per cohort [18]. Prior VEGF-targeted therapy was not allowed. No objective responses were documented during the first stage in patients with carcinoid tumors and trial accrual was consequently stopped. Objective response rate, however, is not an appropriate endpoint for this type of drugs in general, and for this tumor type in particular. Certainly, a cytostatic effect cannot be ruled out with this study design. Median PFS for this cohort was 12.7 months. Among PNET patients, objective responses were observed in 17 % with a median PFS of 11.7 months. A subsequent study by the Spanish Neuroendocrine Tumor Group (GETNE) is currently assessing safety and efficacy of pazopanib in patients with progressive advanced NETs [19]. The major difference with the prior one is that this trial did allow prior targeted therapy with antiangiogenic agents or mTOR inhibitor, and of note, 83 % of included patients (N = 44) had been pretreated with at least one of these agents. The primary endpoint was clinical benefit rate (CBR = CR + PR + SD) at 6 months according to RECIST v1.0 criteria. A number of potential predictive biomarkers are also being evaluated, including VEGF and soluble VEGFR-2 plasma levels, as well as circulating tumor and endothelial cells. Preliminary analysis were recently presented at ESMO 2012 and reported a CBR of 100 % in patients with no previous targeted therapy (7 pts), 89 % in patients previously treated with mTOR inhibitors (9 pts), 83 % in those pretreated with antiangiogenics (12 pts), and of 60 % in patients that had received both antiangiogenics and mTOR inhibitors prior to study entry (5 pts). Grade 3–4 toxicities included asthenia (18 %), hypertension (9 %), diarrhea (9 %), and ALT elevation (11 %). These encouraging results suggest a role for sequential targeted agent therapy in this disease.
Other less successfully tested antiangiogenic agents include Vatalanib, Thalidomide, Atiprimod, and Endostatin. Vatalanib is an orally administered small molecule targeting VEGFR-1,2,3, PDGFR-β, and cKIT. Two small phase II trials with this drug showed limited efficacy with an unfavorable toxicity profile (liver toxicity, dizziness, emesis, hypertension, and proteinuria); and therefore, its development in NETs has been halted [20, 21]. Thalidomide is an agent with antiangiogenic properties of unknown mechanism of action that has proven to be active against multiple myeloma. In NETs, it has been tested in combination with temozolomide [22]. Although results of this phase II trial were encouraging—a radiological response rate of 25 %, a biochemical response rate of 40 % and an overall survival at 2 years of 61 %—it is difficult to estimate the individual contribution of each of these agents to the overall outcome. The combination was tolerable, being the most common toxicity lymphopenia with opportunistic infections documented in 10 % of treated patients. Atiprimod is a JAK2/JAK3 small molecule inhibitor with significant antiproliferative, antiangiogenic, and proapoptotic effects in preclinical models. Atiprimod inhibits the phosphorylation of signal transducer and activator of transcription 3 (STAT3), blocking the signaling pathways of interleukin-6 and VEGF and down regulating the antiapoptotic proteins Bcl-2, Bcl-XL, and Mcl-1, thereby inhibiting cell proliferation, and inducing cell cycle arrest and apoptosis. Preliminary results of a phase II proof-of-concept study of atiprimod in patients with advanced low—to intermediate-grade neuroendocrine carcinomas reported 91 % stabilizations in the 23 patients that completed 2 cycles of therapy with tolerable toxicity profile [23]. Finally, Endostatin is a 20 kd fragment derived from the C-terminal region of mouse collagen XVIII, an extracellular matrix heparin sulfate proteoglycan that is an abundant constituent of blood vessels and most basal laminae in organs distributed throughout the body. Treatment with recombinant murine endostatin induced the regression of experimental tumors growing in mice to dormant, microscopic lesions. The antiproliferative activity of endostatin seems to result specifically from effects directed against endothelial cells. Preclinical studies showed promising antimetastatic and growth inhibitory activity against several tumor models with no discernible host toxicity, and a minor durable response was observed in a patient with a non-functioning pancreatic NET treated with endostatin in the first-in-man phase I clinical trial [24]. Based on this, a phase II trial was conducted in 42 patients with advanced pancreatic NETs and carcinoid tumors [25]. No objective radiological responses were however documented, and the authors concluded that this agent did not have significant antitumor activity in these patients.
More recently, Axitinib, a potent inhibitor of VEGF receptors 1, 2, 3, PDGFR and cKIT, with promising preliminary activity against several vascular-dependent solid tumors, is also undergoing clinical evaluation in NETs. Indeed, the Spanish GETNE group is currently conducting a randomized double-blind placebo-controlled phase II trial (EUDRACT: 2011-001550-29) that aims to accrue 80 patients with advanced and progressive well-differentiated NETs of non-pancreatic origin. Patients are randomly assigned to receive Octreotide LAR in combination with either Axitinib or Placebo. Prior therapy with angiogenesis inhibitors is not permitted. The primary endpoint of the study is PFS. Plasma samples and paraffin-embedded tumor tissue will be collected from all patients to explore the potential prognostic and predictive value of different intracellular pathways involved in VEGFR, PDGFR, and other related RTKs signaling. As of February 2013, 43 patients have been enrolled in the trial, complete accrual is expected within 1 year, and the first interim analysis is preplanned to be performed 6 months following enrollment of 50 % of the study population (that is by August 2013).
Role of the Mammalian Target of Rapamycin Pathway in NETs
Mammalian target of rapamycin (mTOR) is a serine–threonine kinase that plays a key role in regulation of cellular metabolism, growth, and proliferation. mTOR integrates multiple upstream signals including growth factors and mitogens, and active signaling results in an increase in translation of proteins that are important in regulating cell cycle progression and metabolism. mTOR is also involved in angiogenesis control by regulating the translation of hypoxia-inducible factor 1α (HIF1α). In the setting of reduced nutrients or other cellular signals to limit growth, mTOR is inhibited and this leads to increased levels of CDK2 and cell cycle inhibition. The PI3k/AKT/mTOR may be stimulated by upstream activation of VEGFR, PDGFR, and Insulin growth factor receptor (IGFR) and is under negative control of two tumor suppressor genes, tuberous sclerosis 2 (TSC2), and phosphatase and tensin homolog (PTEN). Up to 50 % of human cancers present aberrant activation of this pathway through several mechanisms, including overexpression or amplification of growth factor receptors, activating mutations of the pathway kinases (PI3K, AKT) or loss of function of inhibitory proteins (PTEN, TSC2). In particular, several genetic disorders that have constitutive activation of this pathway, such as multiple endocrine neoplasia type I (MEN1), Von Hippel–Lindau (VHL) or tuberous sclerosis complex (TSC), are associated with an increased incidence of NETs. Loss of TSC2 or PTEN expression reduce the inhibition of mTOR activity caused by hypoxia, and increases the survival and growth of hypoxic tumor cells thus contributing to tumor progression [26]. Autocrine activation of the mTOR signaling pathway mediated through IGF-1 has been implicated in the proliferation of PNET tumor cells. On the other hand, expression profiling assays have shown that TSC2 and PTEN are commonly downregulated in PNETs, and this is inversely correlated with prognosis. Further confirming the relevance of this pathway in the pathogenesis of NETs is the work by Jiao and colleges, which determined the exomic sequence of ~18,000 protein-coding genes in a Discovery set of ten well-characterized sporadic PNETs, and then screened the most commonly mutated genes in 58 additional PNETs [27]. Among others, they found mutations in genes in the mTOR pathway in 14 % of the tumors, including PTEN, PIK3CA, and TSC2. Consistent with these observations, inhibition of mTOR has a significant antiproliferative effect on NET cell lines. All these findings suggest that molecular tumor profiling could potentially play a role for the selection of patients most likely to benefit from mTOR-targeted therapy [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree