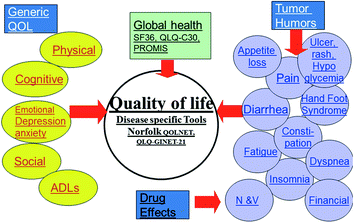
Fig. 14.1
Distribution and frequency of NETs
It is clear that both patients and practitioners need a heightened awareness of the symptoms of the condition that can often masquerade as other disease states (Fig. 14.2). Early recognition, together with the advent of new approaches to therapy—the use of somatostatin analogs alone and in combinations with other chemotherapeutic, surgical, and advanced technological procedures—has had a very significant impact on the course of the disease, which we now may regard as chronic rather than a rapidly progressive and fatal condition. In this milieu, there was a need for developing a questionnaire to capture patients’ responses, that would be able to help define those patients who have the condition while excluding those without it, and have the ability to distinguish between the impact of the disease itself on QoL as opposed to the effects of various intervention and drugs used as therapy.
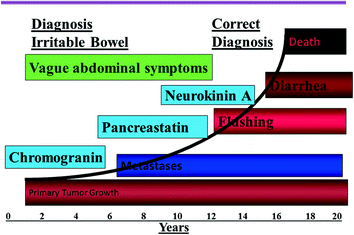

Fig. 14.2
Natural history of NETs and the appropriate diagnostic, staging, and prognostic biomarkers. Modified from Vinik and Moattari [4]
With regard to therapy, a fundamental objective of any health care intervention is the enhancement of the patient’s QoL and overall well-being. A patient’s health-related QoL encompasses their experience as a result of the underlying condition, their response to medical treatment, and consequently how their illness impacts their overall well-being [18]. Consideration of a patient’s QoL has become increasingly important in evaluating the adverse health effects resulting from chronic illnesses such as NETs. Knox et al. [19] found that advanced therapy like surgical resection for NETs is associated with a significantly improved and sustained functional QoL. On the contrary, QoL may be severely impaired by the effects of many chemotherapeutic agents causing nausea, vomiting, and fatigue. The effects of radiation, too, can compromise QoL (Fig. 14.3).
In evaluating QoL, one needs to balance the impact of an intervention with of the impact of the underlying disease. The patient should actively participate in a decision to embark on therapy giving due consideration to the potential negative side effects as a result of the intervention versus any known benefit of the intervention itself. Remaining life, in terms of not only time but also quality, should be evaluated. QoL is a powerful tool to empower patients and their healthcare providers to share in this vital aspect of improving health outcomes.
Comparison of QoL in Patients with NETs and the General Population
Beaumont et al. [2] evaluated the HRQoL in patients with NETs and determined the association with demographic and clinical features of these tumors. Patients with NETs were invited to complete 2 standardized generic measures of QoL including Patient-Reported Outcomes Measurement Information System (PROMIS)-29 and the SF-36 with a set of standard demographic and disease-related questions. General linear models were used to evaluate the associations between HRQoL and demographic and clinical characteristics. They entered a total of 663 patients who demonstrated worse HRQoL when compared to the general population and to a sample of mixed cancer patents and survivors. Patients with a current NET, either not surgically removed or recurrent after surgery, and patients with carcinoid symptoms (flushing and diarrhea) experienced a worse total QoL as well as impaired physical function, social activity, limitation of their physical role, depression, fatigue, pain interference with life, general health, and vitality using the combination of the PROMIS-29 and SF-36 tools (Fig. 14.4).
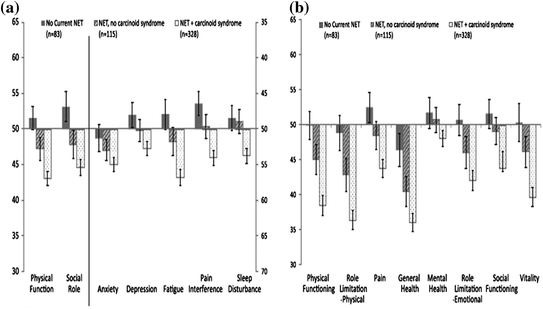

Fig. 14.4
Comparison of HRQoL in patients with neuroendocrine tumors compared with the general US population [2]
This study clearly illustrates that patients with NETs have a worse QoL than the general US population including patients with other small bowel neoplasms. Furthermore, patients with carcinoid syndrome fared worse than patient’s with non-functioning tumors. The greatest limitation of the study was that it was cross-sectional, observational of a non-probability-based study sample. Objective information on tumor burden and biochemical markers was not available, and therefore, no clinical correlations could be derived. Furthermore, the prognostic and discriminatory capacity of the tools could not be assessed with a control population and of the group of subjects followed longitudinally. While this study clearly showed the impact carcinoid syndrome has on general QoL, it fell short and could not define the health relatedness of the various features, for example depression, physical function, and fatigue. This will be explored further below.
Patient-Reported Outcomes in Clinical Trials Using Generic Tools
In the current patient-centered environment, there has been an increasing interest in incorporating patients’ assessments of their health status, giving rise to questionnaires designed to collect and analyze patient-reported outcomes (PROs).
The abbreviation “QoL” used in this chapter will denote HRQoL. Thus, subjective, self-reported patient’ assessments of their health status as it affects their QoL reflect health outcomes related to QoL. Although QoL as a marker for health outcomes is of newly recognized value, the importance of QoL measures for evaluating results of clinical research is indisputable. Clinical trials for a new therapy will not pass through the FDA without the use of a suitable validated HRQoL instrument to assess patient-reported outcomes. QoL measures are also used to discriminate the presence or absence of a condition, discriminate the different levels of severity within a condition, correlate subjective and objective measures, and, most importantly, monitor patient progress. QoL questionnaires administered to patients may help to bridge the gap between patient and physician and may also serve to help touch on issues too sensitive for the patient to address personally. As reported by Clauser et al. [20], patient-reported outcomes are used in a variety of cancer clinical trials to better understand the burden of cancer and the adverse effects of cancer therapy such as pain, fatigue, and nausea. Also mentioned in this article is the fact that the evolution of patient-reported outcomes in cancer trials has been documented by the National Cancer Institutes (NCI)-supported Cancer Outcomes Measurement Working Group. In their endeavor to measure QoL across a wide range of cancers and other diseases, the NCI created the PROMIS [21].
In Europe, the European Organization for Research and Treatment of Cancer (EORTC) developed the first generation of a core questionnaire in 1987 for the measurement of patient-reported outcomes in cancer clinical trials, EORTC QLQ-C36 [22]. Subsequently, EORTC QLQ-C36 was modified to EORTC QLQ-C30, and then again to EORTC QLQ-C30 (version 3.0) in December 1997, and is now the recommended version for new studies. While there was a concerted effort in Europe and later in the USA toward the development of cancer-related questionnaires, no specific tool was available for assessing subjective QoL outcomes in patients with NETs. In fact, it was recognized that although the EORTC QLQ-C30 was an important tool to measure generic aspects of cancer, it had limitations for capturing specific aspects of cancer-related diseases. This lack of disease-specific self-reported QoL measures motivated the development of disease-specific modules.
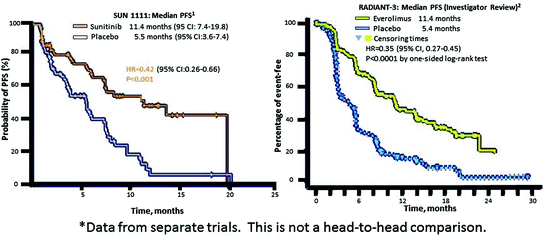
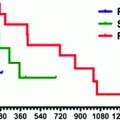
The first randomized, controlled pancreatic neuroendocrine tumor (pNET) trial to include QoL assessment was the phase III study of sunitinib [1, 5] (Fig. 14.5). The study used the EORTC QLQ-C30, a well-validated general oncological HRQoL instrument suitable for a clinical trial setting, but not specific to pNETs.
The QLQ-C30 is composed of both multi-item scales (we also refer to these as domains) and single-item measures. These include five functional scales, three symptom scales, a global health status/QoL scale, and six single items (five of which are also symptoms). Each of the multi-item scales includes a different set of items, and no item occurs in more than one scale. Each domain/scale/item is independent. For all domains/scales/items (with the exception of global HRQoL), patients are asked a series of questions regarding their status over the past week and they respond to one of four choices (a: Not at All; b: A Little; c: Quite a Bit; d: Very Much). There is no “total” score across any of the domains/scales/items.
In interpreting the scores, high scores for a functional domain/scale and the global health status represent a high or healthy level of functioning, while a high score for a symptom scale/item represents a high level of symptomatology or problems. Subjects were included in this study if they completed their baseline EORTC QLQ-C30 assessment and at least one additional post-baseline assessment while on treatment.
Assessments of QoL measures were made every 4 weeks, and the rate of compliance was >80 % (73 of 86 patients in the sunitinib group and 71 of 85 patients in the placebo group). No differences were found between sunitinib and placebo on the cognitive, emotional, physical, role, social functioning, and symptom scales, with the exception of diarrhea, which was significantly worse for sunitinib patients.
Having seen the efficacy data, as well as the results from the repeated measures mixed-effects model (which was comparable across the sunitinib and placebo arms), a post hoc analysis was performed to understand how sunitinib fared in delaying deterioration in global HRQoL and functioning scales. Given that deterioration can be comprised of several factors, for this study deterioration was described as a composite endpoint—death, or first progression, or two consecutive cycles of clinically significant change in a specific HRQoL scale.
Note Given that the 15 scales of the EORTC QLQ-C30 (whether multi- or single items) are independent and cannot be summed up for a total score, each HRQoL scale was evaluated separately as part of the composite endpoint (e.g., death, PFS or global HRQoL; death, PFS or physical functioning).
In order to understand the effect attributable to PFS and death, a sensitivity analysis was carried out by controlling for these two variables by evaluating time to deterioration for the HRQoL scale alone (Tables 14.1, 14.2, 14.3, 14.4).
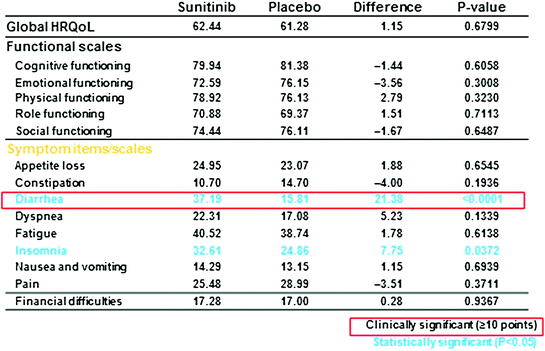
Table 14.1
Patient-reported outcomes assessment
– Global HRQoL |
– Functional scales |
Cognitive, emotional, physical, role and social functioning |
– Symptom items/scalesa |
Appetite loss, constipation, diarrhea, dyspnea, fatigue, insomnia, nausea and vomiting, pain |
• Patients completed the questionnaire at baseline (Cycle 1, Day 1), and Day 1 of every cycle thereafter (cycle = 4 weeks), and at the end of treatment or withdrawal |
Table 14.2
A priori statistical analysis: EORTC QLQ-C30
– Clinical significance (MID): ≥10 points in mean change from baseline |
– Statistical significance: 0.05 level based on a two-sided test |
• Repeated measures mixed-effects models were used as the primary model for between-treatment comparison |
• Descriptive statistics were used for the observed mean and mean change from baseline, and for the proportion of patients who improved or worsened (clinically significant), or who remained stable |
Table 14.3
Baseline EORTC QLQ-C30 scores were comparable with no clinically significant differencesa
Sunitinib (N = 73) | Placebo (N = 71) | |
|---|---|---|
Global HRQoL | 67.0 (62.0, 72.0) | 64.0 (58.4, 69.6) |
Functional scales | ||
Cognitive functioning | 87.1 (83.0, 91.1) | 87.1 (82.6, 91.6) |
Emotional functioning | 75.2 (69.6, 80.9) | 73.7 (67.3, 80.2) |
Physical functioning | 83.1 (78.1, 88.1) | 83.1 (78.0, 88.1) |
Role Functioning | 84.3 (78.6, 90.0) | 77.5 (70.7, 84.3) |
Social functioning | 79.4 (72.7, 86.0) | 77.0 (69.3, 84.8) |
Symptom items/scales | ||
Appetite loss | 16.9 (10.7, 23.1) | 18.7 (11.9, 25.4) |
Constipation | 14.9 (8.9, 21.0) | 14.9 (9.0, 20.7) |
Diarrhea | 20.4 (13.2, 27.6) | 19.2 (12.3, 26.1) |
Dyspnea | 15.9 (10.7, 21.1) | 19.7 (12.5, 26.9) |
Fatigue | 29.4 (23.6, 35.1) | 34.5 (28.0, 41.0) |
Insomnia | 25.4 (18.1, 32.6) | 26.3 (19.4, 33.1) |
Nausea and vomiting | 6.7 (3.7, 9.7) | 12.6 (7.2, 18.1) |
Pain | 22.9 (16.9, 28.8) | 22.5 (15.4, 29.6) |
Financial difficulties | 19.9 (12, 27.8) | 15.7 (8.9, 24.4) |
Table 14.4
Results of a priori analysis: overall post-baseline EORTC QLQ-C30 scores (mixed-effects model) [5]

Patient Disposition
PRO Completion Rate Within the Phase III Study Exceeded 80 % of Patients Eligible for Completing the QLQ-C30 at Each Cycle Within the First 10 Cycles
In a priori analyses, sunitinib was associated with a clinically and statistically significant worsening of diarrhea (diff. = 21.38; P < 0.001) and a statistically significant trend toward worsening of insomnia (diff. = 7.753, P = 0.0372) compared with placebo. However, sunitinib did not differ from placebo in any of the functioning scales (cognitive, emotional, physical, role, social), other symptoms, or in global HRQoL.
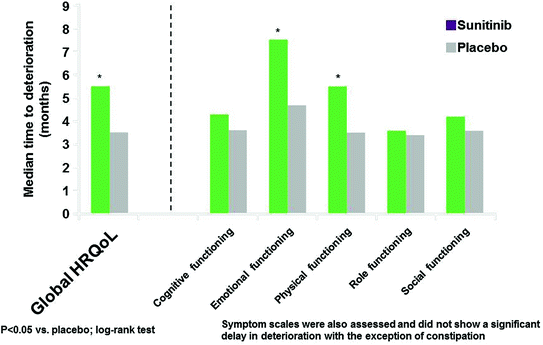
In a post hoc analysis, sunitinib significantly delayed deterioration in two of the five functioning scales (emotional and physical) and global HRQoL based on the composite endpoints of PFS, death, or MID. Figure 14.6 presents the results from the log-rank test comparing TTD between the two treatment arms in global HRQoL and functioning scales.
The vertical axis represents the median time to deterioration in months. There was a statistically significant difference in TTD for global HRQoL, emotional and physical functioning scales favoring sunitinib. This means that sunitinib delayed deterioration in these scales, where deterioration was evaluated as the composite endpoint of PFS, death, or MID. TTD was also evaluated in the symptom scales, and there was not a statistically significant delay in deterioration, with the exception of constipation (in favor of sunitinib) [5].
In a separate study, QoL was evaluated with EORTC QoL 30 in 265 patients with gastroenteropancreatic or bronchial NETs treated with [177 Lu-DOTA, Tyr3] octreotate [26]. Regardless of the treatment outcome, insomnia, appetite loss, and diarrhea improved significantly. Patients with bone metastases or a decrease of 50 % or more in the biomarker chromogranin A (CgA) had improvement in their EORTC scores by at least 10 points. In a subgroup of patients (36 %) with decreased GHS/QoL or symptoms at the start of therapy GHS/QoL improved: Fatigue in 49 %, nausea in 70 %, vomiting in 53 %, pain in 44 %, dyspnea in 59 %, insomnia in 63 %, appetite loss in 60 %, constipation in diarrhea 67 % [26].
Development of Two Disease-Specific Questionnaires
Health-Related QoL in Patients with NETs
As novel treatments are prolonging survival, NETs have become a chronic condition for many patients, making QoL an increasingly important consideration. Patients with NETs have a significantly worse QoL than the general population [27–30]. The symptoms of functioning pNETs, such as diarrhea, rash, and sweating, the toxicity associated with chemotherapy and radiation, and the emotional, social, and cognitive impact of the disease can have a profoundly negative effect on a patient’s QoL. Measurement of QoL using questionnaires to assess PROs in the setting of clinical trials is an important means of evaluating the benefit of treatments. PROs help to bridge the gap between the patient and physician, especially regarding sensitive personal issues related to treatment. Measurement of PROs in cancer trials has become a priority in the USA and Europe. Instruments include the National Cancer Institute’s PROMIS and the European Organization for Research and Treatment of Cancer’s EORTC QLQ-36 [24]. Questionnaires developed specifically to assess health-related QoL in NET patients include the Norfolk QoL-NET and the EORTC QLQ-C30 GI.NET21 [6, 20]. Until recently, only a limited body of research on carcinoid and NETs existed, and as previously mentioned, there was no disease-specific tool to measure health-related QoL in patients with this disease. To fill this need, two questionnaires were simultaneously, but independently developed—on two different continents—to measure the subjective, self-reported effects of NETs on QoL. While there are some distinct similarities, there are also notable differences in each; however, both have the common goal of measuring QoL. The Norfolk QoL-NET, a 72-item all-inclusive single questionnaire was developed in 2004, at the Neuroendocrine Unit, a department within Eastern Virginia Medical School (EVMS), located in Norfolk, Virginia. The development process, which extended over 3 years in different patient populations in the United States, has been described and published [6]. In the interim, the European group working in the NET field also developed a 21-item disease-specific QoL questionnaire to supplement the EORTC QLQ-C30 (their generic cancer measure updated in 1995), mentioned above [31]. They named the new, disease-specific tool, EORTC QLQ-GI.NET21 [22].
Description of Two Disease-Specific Questionnaires
The Norfolk QoL-NET is a fully validated 72-item questionnaire, with excellent internal consistency [6]. It covers seven domains: [1] depression, [2] flushing, [3] respiratory, [4] gastrointestinal (GI), [5] cardiovascular, [6] physical functioning, and [7] positive attitude. Measurements are related to a 4-weeks time frame and capture 11 symptoms, and it measures both frequency and severity of symptoms: flushing, joint/bone pain, other pain, peripheral edema, wheezing, diarrhea/constipation, rash, cyanosis, telangiectasia, fatigue, and coughing. The questionnaire assesses the impact of these symptoms on daily activities, including work, family life, and psychosocial activities. We have shown that the burden of disease plays a major role on physical functioning and consequently on QoL [32]. Twenty questions in the Norfolk QoL-NET were designed to capture activities of daily living and physical functioning.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree