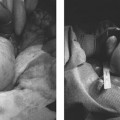
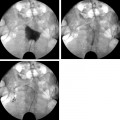
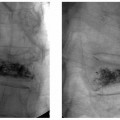
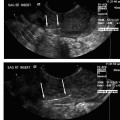
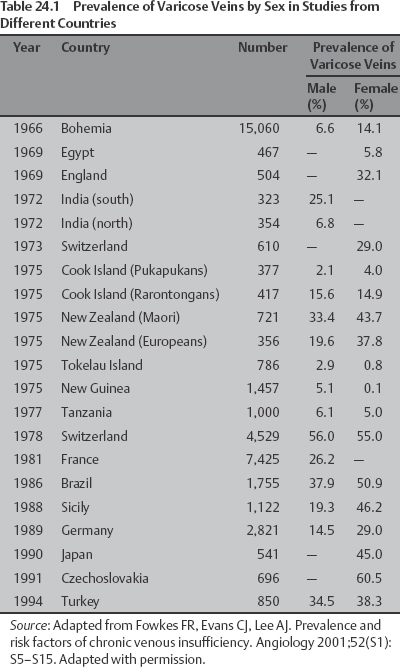
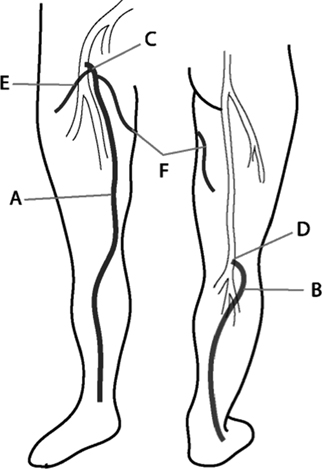
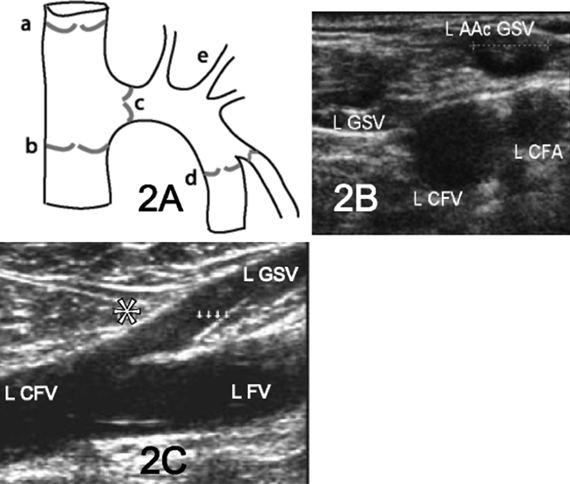
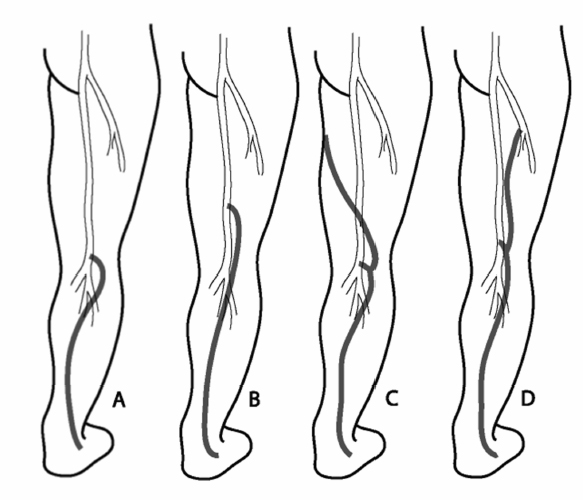
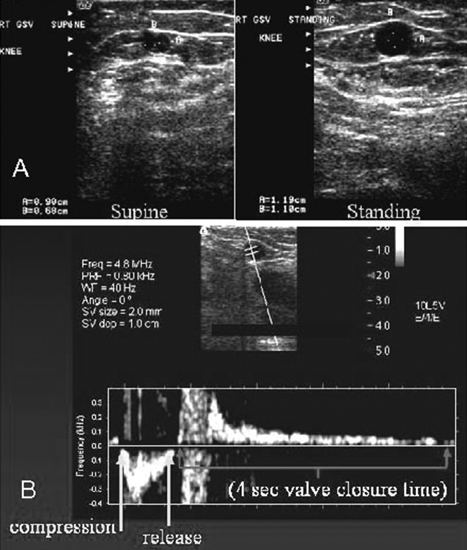
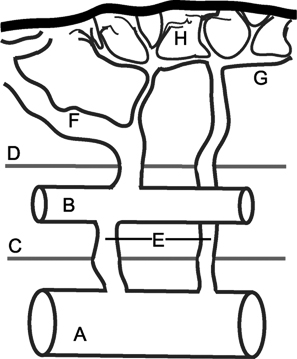
24 Clinical Review: Lower Extremity Venous Insufficiency Chieh-Min Fan Venous insufficiency is one of the most common medical problems affecting the adult population, and is particularly common in women who have two to three times the risk of developing varicose veins as compared with men. Varicose veins are characterized as either primary (developing de novo) or secondary (the sequelae of traumatic or post-thrombotic vein damage). This chapter will review venous anatomy, epidemiology, and pathophysiology of lower extremity venous reflux, as well as the clinical evaluation of patients with varicose veins and venous insufficiency. Lower extremity chronic venous insufficiency (CVI) and varicose veins are among the most common diseases affecting the adult population, estimated to be the seventh most common indication for medical referral in the United States.1 Despite numerous epidemiological studies, the overall prevalence of CVI is difficult to pinpoint given differences in study design and study populations. In a review of 21 epidemiological studies of varicose veins, Callam et al concluded that the overall prevalence of tortuous visible varicose veins in a Western population greater than 15 years of age was 10 to 15% for men and 20 to 25% for women. Table 24.1 presents the prevalence of varicose veins by gender as seen in numerous international epidemiological studies.2 The development of varicose veins is a multifactorial process, likely reflecting an underlying genetic predisposition for venous insufficiency exacerbated by extrinsic factors. Risk factors that have been implicated in the genesis of primary venous insufficiency and varicose veins include positive family history, increasing parity, increasing age, female gender, obesity, and employment or lifestyle involving prolonged standing or sitting. Of these risk factors, genetic predisposition is clearly one of the most significant. In a study of 67 subject/parent sets compared with 67 control subject/parent sets, Cornu-Thenard et al observed that the presence of varicose veins in one parent was associated with a 25% risk of varicose veins in male offspring and 62% risk in females, and if both parents manifested the disease, the risk in offspring increased to 90%.3 Increasing age and lifestyle are both risk factors for varicose vein formation. In the Tampere study4 in which 3284 men and 3590 women were divided into cohorts of 40, 50, and 60 year olds, the overall prevalence of varicose veins in these age groups were 22, 35, and 41% respectively. Lifestyle appears to be potentially etiologically significant in that the Edinburgh, Framingham, and Tampere studies all detected an increased prevalence of varicose veins in individuals employed in occupations requiring prolonged periods of standing as compared with individuals employed in occupations that did not.4–11 Female gender and increasing parity have both been implicated as risk factors for varicose veins. In the Tampere study,1 the risk of women developing varicose veins after 0, 1, 2, 3, or 4 or more pregnancies was 32, 38, 43, 48, and 59%, respectively. Varicose veins are hormonally sensitive to estrogen and progesterone, and increased estradiol levels have been shown to correlate with increased venous distensibility and varicose veins.12,13 Hydrostatic effects of pelvic venous compression by the gravid uterus may compound the hormonal effects upon the veins, resulting in increased risk for lower extremity varicose vein formation. Overall lifetime exposure to these female reproductive hormones may contribute to the higher prevalence of varicose veins in women in general. Due to a lack of official guidelines, the nomenclature of the venous system of the lower extremity was historically inconsistent, resulting in widespread application of variable terminology and confusing abbreviations in the discussion of venous structures in the medical literature. In response to this problem, in 2002, an international interdisciplinary committee convened to standardize the venous nomenclature, resulting in publication of a consensus document that currently defines the accepted formal nomenclature of lower extremity venous anatomy. An additional update with refinements of terminology was published in 2005, and together these two documents define the currently accepted nomenclature of the lower extremity venous structures. Highlights of this consensus opinion are presented below.14–16 Fig. 24.1 Schematic representation of the superficial venous system of the lower extremity. (A) Great saphenous vein (GSV). (B) Small saphenous vein. (C) Saphenofemoral junction. (D) Saphenopopliteal junction. (E) Anterior accessory of the GSV. (F) Posterior accessory of the GSV. The venous anatomy of the lower extremity consists of large capacitance deep venous system in the core of the leg, and a smaller capacitance superficial venous network near the surface. Technically, the superficial venous system also includes the epigastric veins and veins of the external genitalia. The two venous systems work together to carry the venous blood return from the extremity back to the central circulation. The deep and superficial systems connected at several consistent points: the saphenopopliteal junction (SPJ), the saphenofemoral junction (SFJ), and through an extensive system of horizontal bridging perforator veins. Anatomic studies estimate the presence of ~60 to 100 perforator veins in the lower extremity, which were historically grouped and named after eminent vascular surgeons, Hunter, Dodd, Boyd, and Cockett. These eponyms have been replaced by more anatomically descriptive terminology.14–16 The SFJ and SPJ are commonly implicated as primary sites of origin for venous reflux in the formation of varicose veins and also represent the central extension limits of endovenous saphenous thermal ablation. As such, these major deep-to-superficial connections merit additional discussion. The SFJ is bounded superiorly by the suprasaphenous valve and inferiorly by the infrasaphenous valve of the femoral veins. The SFJ also includes the GSV segment defined by the preterminal valve and terminal valves between which the tributary branches enter and drain (Fig. 24.2).15 The SPJ represents the junction of the SSV to the popliteal vein, and has several common variants. The SSV may terminate directly in the popliteal vein, or commonly more superiorly at the suprapopliteal level. The SSV can also continue superiorly as the superior extension of the SSV and intersaphenous vein (formerly the Giacomini vein) which forms a direct connection to the posterior accessory of the GSV. Infrequently, the SSV may terminate directly into the deep femoral vein (Fig. 24.3).17 Fig. 24.2 Anatomy of the saphenofemoral junction (SFJ). (A) Schematic representation of the SFJ which is bounded superior by the suprasaphenous valve (a), inferiorly by the infrasaphenous valve (b), and within the great saphenous vein (GSV) by the preterminal valve (d) and terminal valve (c).15 (B) Axial ultrasound image of the left (L) saphenofemoral junction demonstrating the relationship of the L GSV to the L anterior accessory GSV (L AAc GSV), common femoral vein (L CFV), and common femoral artery (LCFA). (C) Longitudinal ultrasound image of the left SFJ (asterisk) showing the relationship of the L GSV to the femoral vein (L FV) and common femoral vein (L CFV). Fig. 24.3 Anatomic variants of the saphenopopliteal junction. (A) Most common pattern with short saphenous vein (SSV) insertion into the midpopliteal segment. (B) Superior extension of the SSV with high insertion into the upper popliteal/lower femoral vein. (C) Continuation of the superior extension of the SSV as the intersaphenous vein (formerly Giacomini vein) to anastomosis with posterior accessory of the great saphenous vein. (D) Uncommon variant in which the superior extension of the SSV enters into the deep femoral vein. Fig. 24.4 Ultrasound evaluation of saphenous vein reflux. (A) Axial ultrasound images of the proximal great saphenous vein (GSV) in a patient with saphenous reflux. Note the marked dilatation of the GSV in the standing position. Also note characteristic “Egyptian eye” appearance of the GSV as it travels between the superficial and deep layers of fascia. (B) Spectral Doppler analysis of GSV reflux using calf compression. Compression is applied and then released (arrows), resulting in reversal of flow (reflux). The duration of the reflux is 4 seconds (passive valve closure time). Fig. 24.5 Schematic representation of the cross-sectional distribution of venous structures from subdeep fascia to dermis. (A) Deep vein. (B) Saphenous vein running between the deep fascia (C) and superficial fascia (D). Perforator veins (E) cross the fascial layers to directly connect saphenous and more superficial venous branches to the deep venous system. The saphenous vein joins tributary (F) and reticular branches (G). Local venous incompetence in the reticular network can result in telangiectasia and venulectasia at the skin surface. By definition, both the great and small saphenous veins are contained between superficial and deep fascial layers, and in cross-section have a characteristic “Egyptian eye” configuration (Fig. 24.4). If the saphenous vein crosses the superficial fascia into the subdermal tissues, it technically becomes a tributary vein.18 Tributary veins are the largest nonsaphenous lower extremity branches, and share the suprafascial/subdermal space with a reticular vein network. Perforator veins connect both the saphenous veins and more superficial tributary and reticular venous branches to the deep venous system. Venous reflux in the reticular veins may give rise to venulectatic and telangiectatic patches on the skin surface. Figure 24.5 presents a schematic diagram of the subfascial and subdermal venous structures. Both superficial and deep venous systems work together to carry the venous return from the leg to the central circulation. The venous load is normally distributed asymmetrically, with the deep system carrying ~90% of the volume load versus 10% in the superficial system. Flow direction in the venous system is normally unidirectional toward the heart in the superficial and deep veins, and from superficial to deep through the perforators. Normal venous flow direction is maintained through a system of one-way valves that prevent retrograde venous flow back into the leg(s), and from deep veins to the surface. Intrinsic factors that assist in maintaining venous flow include venous contractions, arterial pressure, muscular contractions, respiratory movements, intrathoracic and intraabdominal pressure, and valve competency. Extrinsic factors include gravity, extrinsic compressive forces, and atmospheric pressure. Of the various factors that contribute to forward venous flow, the primary driving mechanism is contraction of the musculovenous pump of the calf and thigh.19,20
Epidemiology of Varicose Veins
Lower Extremity Venous Anatomy
Nomenclature
Anatomy
Normal Venous Physiology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree