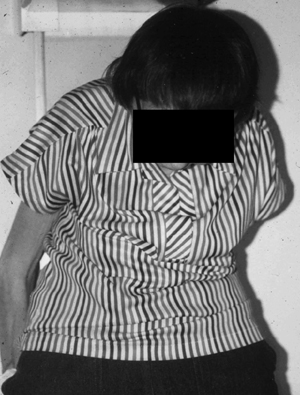
19 Clinical Review: Osteoporosis Michael F. Holick Osteoporosis is the most common metabolic bone disease worldwide.1–4 It is estimated that 25 million Americans are at risk for osteoporosis. Osteoporosis is more common in women than adult onset diabetes, heart disease, and stroke combined. Osteoporosis by definition means that both the matrix and mineral content of the skeleton has declined to the degree where there is loss of architectural integrity increasing risk of fracture. The prevalence of osteoporosis increases progressively with age, and by the age of 80, 25% of women and 15% of men will have had a hip fracture.1–5 Approximately 33% of women 60 to 70 years of age and 66% of those 80 years of age or older have osteoporosis.1–4 Forty-seven percent of women and 22% of men 50 years of age or older will sustain an osteoporotic fracture.2,4 Although loss of bone mineral density (BMD) per se is considered by many to be a benign disease of little consequence, an osteoporotic fracture can cause chronic debilitating pain, diminished quality of life, and even death. Approximately 1.5 million skeletal fractures occur in women annually in the United States, many of which can be attributed to osteoporosis. Two hundred fifty-thousand hip fractures occur annually, and ~20% of these patients will die within the first year from complications of the fracture and 50% never regain their previous quality of life.2,4,5 Approximately 8 to 10 billion dollars are spent annually for the acute care of patients with hip fracture. As the U.S. population ages by 2020, it is estimated that $120 billion dollars a year will be spent for acute and chronic care of these patients.2,3 Classically, osteoporosis is associated with a thin white female who has been postmenopausal for 10 to 15 years (Fig. 19.1). However, all men and women are at risk for developing osteoporosis. Some of the more common causes associated with osteopenia and osteoporosis include early age of onset for menopause, history of amenorrhea or oligomenorrhea during the second and third decades, hypogonadism, family history, poor lifetime intake of calcium, and chronic vitamin D deficiency (Table 19.1).2,4,6 In addition, heavy cigarette smoking, premature graying of the hair (50% of the hair turns gray by the age of 40 years), several endocrinopathies including hyperthyroidism and primary hyperparathyroidism, genetic diseases of collagen synthesis, i.e., Ehlers–Danlos syndrome and osteogenesis imperfecta as well as chronic glucocorticoid and antiseizure therapy are major risk factors for this debilitating disease (Tables 19.1 and 19.2).2,4,6,7 Fig. 19.1 Elderly woman presenting with severe kyphosis consistent with wedge fractures of her cervical and thoracic spine. Her only complaint was that she could not look up any longer. (Copyright Michael F. Holick, PhD, MD, 2007. Reprinted with permission.) The major precipitating cause of osteoporosis in women is loss of ovarian function.2–4 Calcium and vitamin D deficiency in addition to aging will also cause a significant reduction in BMD.2,4,7–9 For men and women, estrogen plays an essential role in bone remodeling.2,4 Osteoblasts that make the collagen matrix for bone mineralization have receptors for estrogen. Estrogen regulates bone mineralization and demineralization by coupling osteoblastic and osteoclastic activity. Estrogen deficiency causes an uncoupling of this process leading to an imbalance resulting in an increase in osteoclastic activity (bone resorption). This ultimately causes the dissolution of the matrix and mineral.
Risk Factors
Pathophysiology
| Early menopause (natural or surgical) |
| Amenorrhea or oligomenorrhea in second and third decades |
| Thin body habitus |
| Family history |
| Inadequate lifelong calcium intake (<800 mg/day) |
| Chronic vitamin D deficiency |
| Hypogonadism |
| Cigarette smoking |
| Premature graying (50% of hair turns gray before age 40) |
| Steroid and antiseizure therapy |
| Excessive thyroid hormone replacement |
| Immobilization |
As women enter menopause, they begin to lose on average 2 to 4% of their bone mass each year. This is unrelenting and continues for at least a decade, causing a 30 to 40% reduction in BMD. Aging also plays a role by decreasing bone mineral density on average of 0.5 to 1% per year after the age of 50 years. Chronic calcium deficiency results in transient decreases in blood ionized calcium levels, which are immediately corrected by increased production of parathyroid hormone (PTH). PTH stimulates the production of osteoclasts that dissolve bone, causing both osteopenia and osteoporosis. Similarly, vitamin D deficiency results in a decrease in intestinal calcium absorption, which leads to a compensatory increase in PTH levels resulting in the same destruction of the skeleton (Fig. 19.2).2,4,7
Calcium, Vitamin D, and Bone Physiology
The body obtains all of its calcium from the diet. The hormone responsible for providing the body with its calcium requirement is vitamin D. Vitamin D coming from either exposure to sunlight or from diet requires two successive hydroxylations. The first occurs in the liver to form 25-hydroxyvitamin D [25(OH)D], the major circulating form of vitamin D. This is the form of vitamin D that is used to measure a person’s vitamin D status. 25(OH)D, however, is biologically inactive and requires activation (hydroxylation) in the kidneys to form 1,25-dihydroxyvitamin D [1,25(OH)2D], the biologically active form of vitamin D (Fig. 19.2).7
1,25(OH)2D production is controlled by serum PTH, calcium, phosphorus, and fibroblast growth factor 23 (FGF-23). Hypocalcemia and hypophosphatemia will stimulate the kidney’s production of 1,25(OH)2D and FGF-23 will reduce it. 1,25(OH)2D interacts with its vitamin D receptor (VDR) in the small intestine to enhance the absorption of dietary calcium and phosphorus (Fig. 19.2).7 Only 10 to 15% of dietary calcium and 60% of dietary phosphorus is absorbed in the absence of vitamin D. 1,25(OH)2D enhances intestinal calcium absorption to 30 to 40% and phosphorus absorption to ~ 80%.7
| Common |
| Vitamin D deficiency |
| Calcium deficiency |
| Estrogen deficiency |
| Testosterone deficiency |
| Thyrotoxicosis (natural or TSH-suppressive doses of thyroxine) |
| Hyperadrenocorticism (Cushing syndrome) |
| Hyperparathyroidism |
| Chronic glucocorticoid and antiseizure medication use |
| Immobilization |
| Less common |
| Malabsorption |
| Vitamin C deficiency (scurvy) |
| Chronic heparin administration |
| Systemic mastocytosis |
| Adult hypophosphatasia |
| Chronic renal failure |
| Primary biliary cirrhosis |
| Cancer |
| Chronic obstructive lung disease |
| Rheumatoid arthritis |
| Inherited |
| Osteogenesis imperfecta |
| Ehlers–Danlos syndrome |
| Marfan syndrome |
| Homocystinuria |
Vitamin D deficiency results in secondary hyperparathyroidism. PTH increases tubular reabsorption of the calcium in the kidneys, stimulates the kidneys to produce 1,25(OH)2D and stimulates osteoblasts through its receptor to increase the expression of receptor activator of NFκB (RANK) ligand (RANKL). The preosteoclast that has the RANK interacts with RANKL on the osteoblast which, in turn, induces it to become a mature osteoclast (Fig. 19.3). Once mature, it secretes hydrochloric acid and variety of enzymes including collagenases to dissolve the bone matrix and mineral to release calcium into the circulation.2,4,7
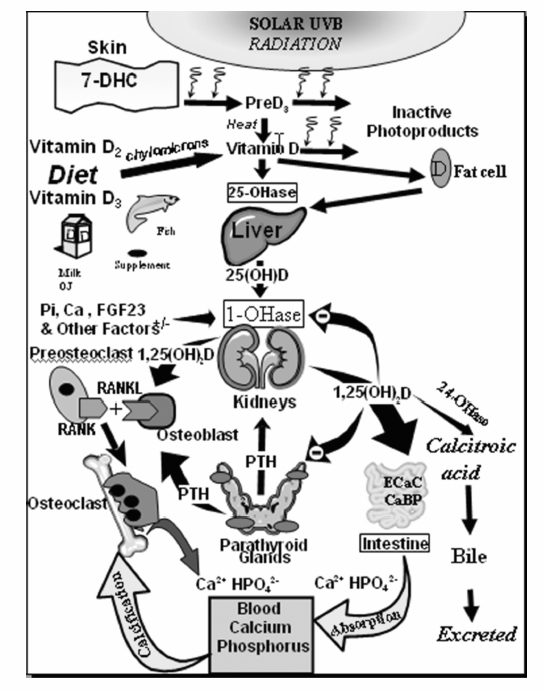
Fig. 19.2 Schematic representation of the synthesis and metabolism of vitamin D for regulating calcium, phosphorus, and bone metabolism. During exposure to sunlight, 7-dehydrocholesterol (7-DHC) in the skin is converted to previtamin D3 (preD3). PreD3 immediately converts by a heat-dependent process to vitamin D3. Excessive exposure to sunlight degrades previtamin D3 and vitamin D3 into inactive photoproducts. Vitamin D2 and vitamin D3 from dietary sources are incorporated into chylomicrons, transported by the lymphatic system into the venous circulation. Vitamin D (D represents D2 or D3) made in the skin or ingested in the diet can be stored in and then released from fat cells. Vitamin D in the circulation is bound to the vitamin D binding protein, which transports it to the liver where vitamin D is converted by the vitamin D-25-hydroxylase (25-OHase) to 25-hydroxyvitamin D [25(OH)D]. This is the major circulating form of vitamin D that is used by clinicians to measure vitamin D status (although most reference laboratories report the normal range to be 20 to 100 ng/mL, the preferred healthful range is 30 to 60 ng/mL). 25(OH)D is biologically inactive and must be converted in the kidneys by the 25-hydroxyvitamin D-1 α-hydroxylase (1-OHase) to its biologically active form 1,25-dihydroxyvitamin D [1,25(OH)2D]. Serum phosphorus, calcium, fibroblast growth factor (FGF-23) and other factors can either increase (+) or decrease (–) the renal production of 1,25(OH)2D. 1,25(OH)2D feedback regulates its own synthesis and decreases the synthesis and secretion of parathyroid hormone (PTH) in the parathyroid glands. 1,25(OH)2D increases the expression of the 25-hydroxyvitamin D-24-hydroxylase (24-OHase) to catabolize 1,25(OH)2D and 25(OH)D to the water-soluble biologically inactive calcitropic acid, which is excreted in the bile. 1,25(OH)2D enhances intestinal calcium absorption in the small intestine by stimulating the expression of the epithelial calcium channel (ECaC; also known as transient receptor potential cation channel subfamily V member 6 [TRPV6]) and the calbindin 9K (calcium-binding protein; CaBP). 1,25(OH)2D is recognized by its receptor in osteoblasts causing an increase in the expression of receptor activator of NFκB ligand (RANKL). Its receptor RANK on the preosteoclast binds RANKL, which induces the preosteoclast to become a mature osteoclast. The mature osteoclast removes calcium and phosphorus from the bone to maintain blood calcium and phosphorus levels. Adequate calcium and phosphorus levels promote the mineralization of the skeleton and maintain neuromuscular function. (Copyright Michael F. Holick, PhD, MD, 2007. Reprinted with permission.)
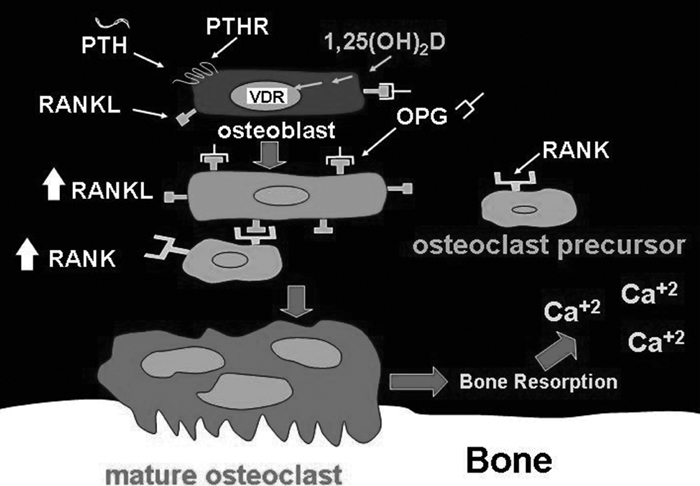
Fig. 19.3 1,25(OH)2D and parathyroid hormone (PTH) stimulation of the mobilization of calcium from the skeleton through interactions with their respective receptors on osteoblasts, which induce expression of the receptor activator of nuclear factor –κB (RANK) ligand (RANKL). The receptor activator of nuclear factor –κB on immature osteoblasts binds to the receptor activator of nuclear factor –κB ligand, which causes the cells to mature and coalesce with other osteoclast precursors to become mature multinuclear osteoclasts. (Copyright Michael F. Holick, PhD, MD, 2007. Reprinted with permission.)
Using Dual Energy X-Ray Absorptiometry
Before the advent of bone densitometry, the radiologist made the diagnosis of osteoporosis either based on the presence of a nontraumatic fracture in the spine or by observing a decrease in the density of the skeleton relative to soft tissue density in an x-ray. However, a patient needs to lose 30 to 50% of the skeletal mass before this can be detected by a radiograph (Fig. 19.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree