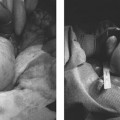
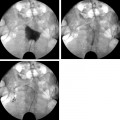
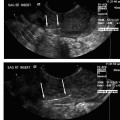
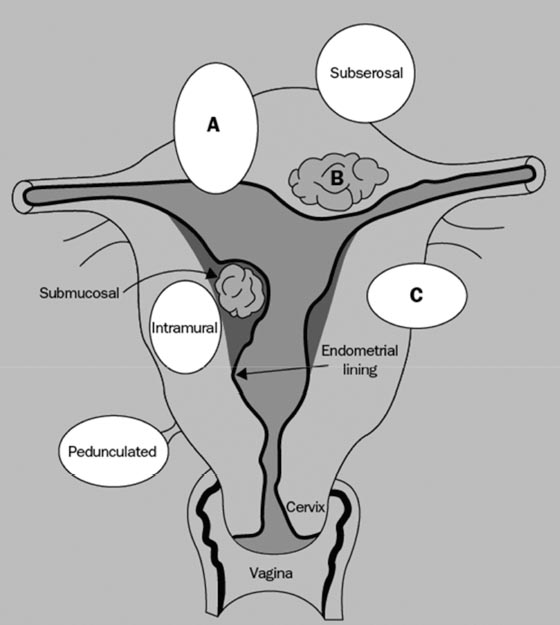
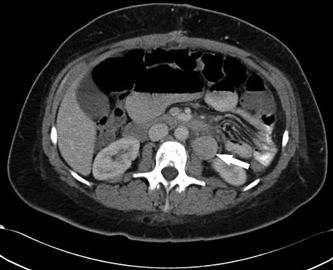
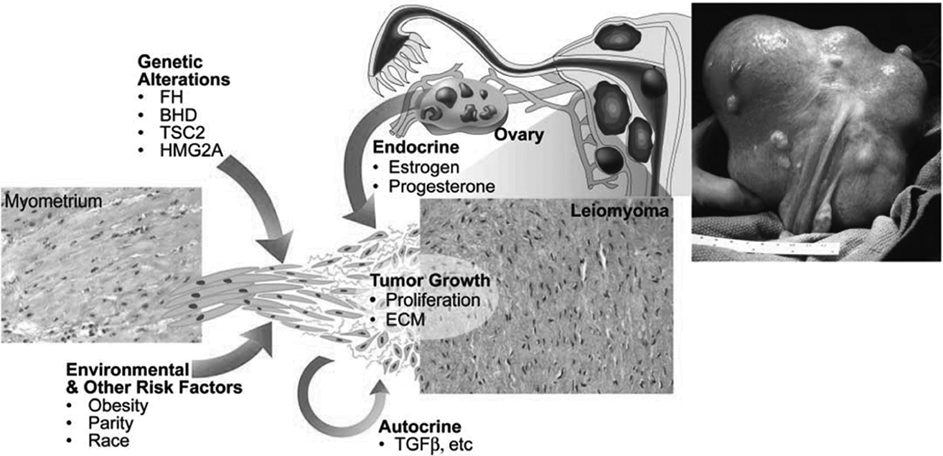
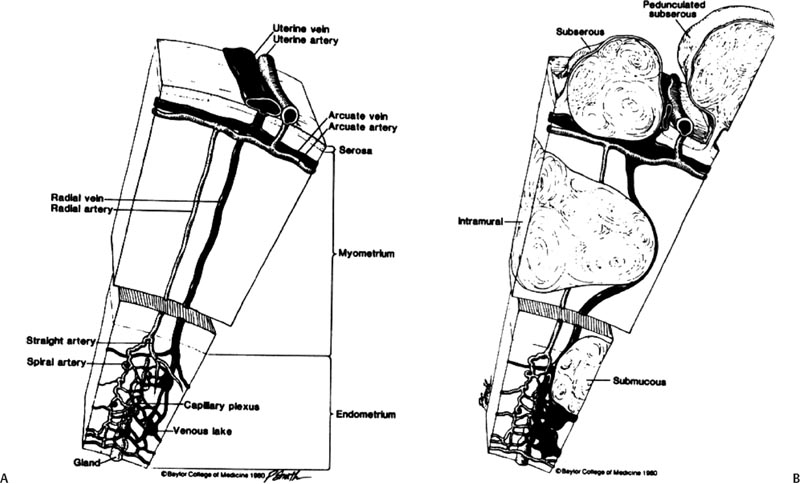
4 Clinical Review: Uterine Leiomyomas Richard Shlansky-Goldberg, Mark Rosen, and Ann Honebrink The term fibroids was introduced by Karl von Rokitansky in 1860 and M. M. Klob in 1863.1 Virchow demonstrated that these neoplasms were derived from smooth cells and introduced the word myoma.1 These benign neoplasms are composed of smooth muscle cells arranged in a whorl-like pattern with a variable amount of collagen, extracellular matrix, and fibrous tissue. They are the most common gynecologic tumor. Leiomyomata are also known as myomas, fibroids, fibromyomas, leiomyofibromas, and fibroleiomyomas.2 The term fibroid is used based on the fibrous tissue demonstrated in these tumors along with the smooth muscle tissue.2 Clinical studies of women during their reproductive age demonstrate a prevalence of 20 to 25%.3 The prevalence is three to nine times higher in black women as compared with white women.4 The data are not as available for other groups, but the rates are similar in white, Hispanic, and Asian women.4 Twenty to fifty percent of uterine leiomyomas are thought to produce symptoms.5–7 Leiomyomas may develop in multiple locations (Fig. 4.1). The severity of symptoms depends on the number, size, and location of these benign tumors.5–7 Fig. 4.1 Leiomyomas may be located in multiple different locations within the uterus. Submucosal leiomyomas extend into the cavity, intramural leiomyomas are contained within the wall of the uterus, and subserosal leiomyomas extend from the uterus surface. Most leiomyomas are mixed such as A, B, and C. Leiomyomas in position A are transmural extending from the submucosa into the serosa. Pedunculated submucosal leiomyomas are intracavitary. (From Stewart EA. Uterine fibroids. Lancet 2004;357:293–298. Reprinted with permission.) It is difficult to predict the biological behavior of uterine smooth muscle tumors. Myomas appear to arise from a single myometrial cell mutation and are often described as clonal.8,9 The vast majority of these neoplasms follow a benign course, but a small number will behave aggressively, exemplified by metastatic disease or recurrence after surgery. Patients after myomectomy have demonstrated a 10-year clinical recurrence rate of 27% using life-table analysis.10 One concern is that if there is rapid growth, there may be an increased risk for malignancy. More recent studies have shown that even with rapid growth, the chance of developing a malignancy in preexisting fibroids remains very low.3,11 The incidence of leiomyosarcoma is estimated to be 0.23% in premenopausal women and peaks at <2% in postmenopausal women.12 The genetic differences between leiomyomas and sarcomas suggest that they develop from different origins and that sarcomas do not result from malignant degeneration of an existing leiomyoma.13 Benign and malignant uterine smooth muscle tumors are cytogenetically distinct.14 More than half of leiomyomas have no discernible karyotypic abnormalities on conventional cytogenetic analysis. With the remainder of these benign tumors having simple karyotypes that have been characterized into, at least, six cytogenetic subgroups, suggesting that multiple genes and mechanisms are involved in the development of these tumors.14 In contrast, leiomyosarcomas have frequent structural and numerous chromosomal abnormalities and karyotypes vary widely among metaphase cells.15 A majority of classification schemes prognosticates the biological behavior of a myoma and relies on mitotic behavior.16 In 1970, Kempson and Bari17 developed a scheme on nuclear atypia and mitotic rate. Although other markers have been evaluated, these seem like the two that are the most useful criteria to establish the aggressiveness of the tumor.16 There are unusual forms of leiomyomas that have both malignant and benign features. These include benign metastasizing leiomyomas (BML), which is characterized by leiomyoma-like lesions in the lungs or abdomen in patients with uterine leiomyomas (Fig. 4.2). Lymphan-gioleiomyomatosis, similar to BML, has a characteristic lung lesion that originates from benign renal angiomyolipo-mas.18 Intravenous leiomyomatosis are hormonally responsive lesions that have vermiform extensions from the uterus, which may extend to the heart but do not metastasize.19 Fig. 4.2 Benign metastasizing leiomyoma. A forty-one year-old woman with prior hysterectomy for leiomyomata, presenting with multiple retroperitoneal masses. Computed tomography (CT) scan at the level of the lower kidneys demonstrates a round uniformly enhancing mass in the paraaortic region (arrow). On biopsy, pathology demonstrated benign metastasizing leiomyoma. Remaining mass was stable at 6-month follow-up imaging. Investigating the racial differences in the incidence of leiomyomas, Kjerulff et al20 reviewed the hysterectomy data for noncancerous diseases for 409 black and 836 white women in 28 hospitals in Maryland. They found that 89% of the black women, compared with only 59% of white women, had leiomyomas. The black women had larger and more numerous leiomyomas. They were also more symptomatic, despite being diagnosed at an earlier age than whites. In reviewing the risks of uterine leiomyomas in black women, Wise et al25 found the presence of leiomyomas were inversely associated with age at menarche, parity, and age at first birth, and positively associated with years since last birth. Increased weight or obesity appeared to attenuate the inverse association between parity and uterine leiomyomas. In general, greater parity decreases the risk of developing leiomyomas.21,22 The use of oral contraceptives in patients with leiomyomas has been controversial in the past. Initially, oral contraceptive therapy was felt to be contraindicated because it was thought that it might stimulate the tumors resulting in an increase in size and numbers. Recent data suggests that oral contraceptives may protect against leiomyoma formation.22–24 Ross et al22 reported a 31% risk reduction in women who had used oral contraceptives for more than 10 years. Some investigators did demonstrate an increase in risk associated with the use of oral contraceptive. Marshall et al4 demonstrated that, when used between the ages of 13 to 16, there was an increased risk of leiomyoma formation. Wise et al25 found an inverse relationship with progestin-only pills when compared with no hormone use and no other consistent patterns of increased growth for other forms of hormonal control. Lower dose oral contraceptives are commonly used currently to treat the heavy bleeding that is often associated with fibroids. Other environmental factors may impact the risk of developing leiomyomas. Smoking has been associated with decreasing the risk of developing leiomyomas.21,22 Consumption of beef and ham has been shown to increase the risk whereas eating green vegetables may decrease the risk.26 Wise and colleagues25 demonstrated an increased risk for women consuming alcohol, but no increase seen in cigarette smokers or caffeine drinkers. There is no evidence that changing dietary habits impacts the growth of fibroids once diagnosed or the risk of developing symptoms.27 Fig. 4.3 The development of uterine leiomyomas is variable in their natural history and etiology. Hereditary genetic defects in several genes (fumarate hydratase (FH); Birt–Hogg–Dubé syndrome (BHD), and tuberous sclerosis 2 (TSC2), as well as somatic changes in the high mobility group AT-hook 2 (HMGA2) genes and risk factors such as obesity, parity, and race participate in the development of leiomyomas. Tumor growth takes place due to an increase in number of cells and extracellular matrix and is influenced by endocrine and autocrine growth factors. (From Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005; 308:1589–1592. Reprinted with permission from AAAS.) Historically, leiomyomas have not been considered a genetic disorder; however, clinical evidence suggests that there may be some genetic component in their etiology.28 Having a first-degree relative with the disease increases the risk of an individual developing leiomyomas and identical twins have a higher concordance than fraternal twins.29–31 Also, patients with the autosomal dominant disease, hereditary leiomyomatosis, and renal cell carcinoma have genetic defects in the fumarate hydratase (FH) gene, which may be involved in the development of these tumors.32,33 Approximately 40% of leiomyomas have an abnormal karyotype with some consistent patterns suggesting that the genetic abnormality may be located in these disrupted regions.8,34,35 Other genes include the tuberous sclerosis 2 (TSC2) gene and the Birt-Hogg-Dube syndrome (BHD) gene due to the increase in leiomyomas in these patients.28 The HMGI-C gene, now called the high mobility group AT-hook 2 (HMGA2) gene, was the first gene identified in chromosomal rearrangements in the development of uterine leiomyomas.36 Potentially, the recognition of different phenotypes and genotypes of specific leiomyomas may have an impact on deciding what therapy to offer patients, if risks such as sarcoma or recurrence can be seen to be associated with specific genetic findings.28 The risk factors associated with leiomyomas are important through their contribution to the initiation or promotion of tumorigenesis. As mentioned above, genetics play an important role. Estrogen and progesterone act as important promoters of uterine leiomyoma growth.37 This is substantiated by the fact that fibroids have not been reported in girls prior to puberty. Most women are diagnosed in their 30s and 40s when their symptoms begin or when fibroids are found incidentally during a routine pelvic exam.27 Estrogen upregulates both the estrogen and progesterone receptors in leiomyomas during the follicular phase of the menstrual cycle, followed by progesterone-induced mitogenesis during the luteal phase.37 In addition, several growth factors are involved in the development of these tumors including transforming growth factor beta (TGF-β), basic fibroblastic growth factor (bFGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF).37 TGF-β and bFGF may be partiality important given their combined mitogenic effect and promotion of extracellular matrix.37 EGF and TGF-β3 are important factors because both have elevated expression during the luteal phase when leiomyoma mitotic activity is greatest. IGF-I has a potent mitogenic effect because both the peptide and its receptors are overexpressed in leiomyomas.37 A summary of the multiple factors involved in the development of leiomyomas can be seen in Fig. 4.3. Most patients with leiomyomas are asymptomatic. When symptoms occur, they are variable, but generally the published data on health-related quality of life (HRQOL) associated with uterine leiomyomas report significantly lower HRQOL scores for women with leiomyomas than for women without this disorder.38 In addition, the health care costs for patients with symptomatic leiomyomas are almost four times higher than those who do not have leiomyomas.39 Symptoms include bleeding, infertility, pain, pressure, urinary frequency, and constipation. As previously mentioned, the degree of symptoms is influenced by the location, size, and number of leiomyomas. In a literature review by Buttram and Reiter, 3 in 1698 patients prior to myomectomy, the incidence of menorrhagia and pain was 30% and 34%, respectively. In a more recent study of patients undergoing uterine artery embolization by Myers et al, the distribution of the predominant symptoms of bleeding, pain, and bulk related was 64.7%, 10.5%, and 23.3%, respectively.40 Several causes of bleeding due to leiomyoma have been suggested. The severity of bleeding may be impacted by the presence of submucosal leiomyomas; the incidence of bleeding is not dependant on this location.3 Sehgal and Haskins41 suggested that the surface area of the endometrium is increased by the presence of submucosal leiomyomas, which provides a greater area to bleed. They demonstrated that the severity of bleeding correlated with the increase in surface area. This hypothesis does not explain the cause of increased bleeding in patients with intramural or subserosal leiomyomas.3 Another mechanism that may explain the cause of bleeding is that leiomyomas may interfere with uterine contraction, which is thought to play a role in the regulation of bleeding.42,43 This theory has not been proven.3 The theory that may best explain the mechanism for bleeding involves the changes in venous structures in the endometrium and myometrium causing venule ectasia.44 This concept was originally described by Sampson in 191245 and supported by Faulkner42 in 1945. Later, light microscopy studies by Farrer-Brown et al demonstrated the venous ectasia.43,46 The original concept was that the leiomyomas compress the venous drainage resulting in the ectasia (Fig. 4.4).3 Current data suggests that the venous ectasia is due to vasoactive growth factors such as bFGF and VEGF.44 These studies also implicate arterial changes that result in an increase in arterial supply to the leiomyomas.44 Fig. 4.4 Anatomical causes of uterine bleeding due to the presence of leiomyomas. (A) Diagram demonstrates the vascular structures of a normal uterus. (B) Diagram of the venule ectasia found in the endometrium caused by the physical obstruction by leiomyoma in the myometrium as proposed by Farrer-Brown.43,46 (From Buttram VC, Jr., Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril 1981; 36:433–445. Reprinted by permission.) Fibroids are felt to be the cause of infertility in less that 1 to 3% of infertile women. However, their role remains a subject of debate.3,47 The primary suspected mechanisms include interference of implantation by altering the endometrial contour, enlargement and deformity or obstruction of the uterine cavity, altered contractility of the uterine inhibiting sperm transport, obstruction of the tubal ostia, and persistence of intrauterine blood or clots.48 In patients with infertility due to leiomyomas, pregnancy rates may be improved by surgical resection.49 In a meta-analysis of 46 articles by Donnez and Jadoul, they determined the pregnancy rate after myomectomy to be 48%, without any significant difference between hysteroscopic myomectomy (45%) and laparoscopic and abdominal myomectomy (49%). Pregnancies occurred quite soon after myomotomy, 7.5 ± 2.6 months.50 Leiomyomas are also associated with an increased risk in placental abruption if the placenta implants over the leiomyoma.51
Racial Demographics and Risk Factors
Genetics
Pathophysiology
Symptoms
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree