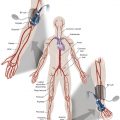
After an arteriography or intervention, the access site must be effectively managed. The planning for access site control starts before gaining access. The operator must make sure that (1) the accessed artery is one that can be compressed, (2) the artery is of adequate size to allow manual compression versus the use of closure devices, and (3) the coagulation status has been taken into consideration.
In addition to the prepuncture planning, the options available for device closure are often defined by the quality of the vascular access. Optimal technique is needed to make sure that the access is centered properly along the vessel and performed at the appropriate level ( Fig. 8.1 ). An access either too high or too low increases the risk of device failure or inadvertent vessel occlusion, respectively.
Manual Compression
Manual compression (MC) has been the gold standard for achieving hemostasis after arterial access for more than 50 years. Manual compression has a high success rate and a low complication rate. Hemostasis can usually be achieved within about 15–25 minutes, although the exact time can vary based on arteriotomy size, health of the vessel, and patient coagulation. Hematoma is the most common complication, although most are minor, requiring only conservative therapy. The major complication rate is approximately 2%, and includes pseudoaneurysm, retroperitoneal hemorrhage, and vessel thrombosis.
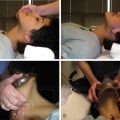
Although MC appears straightforward, there are a few steps that can help direct the operator in an optimal technique for holding pressure (described for common femoral artery access).
- 1.
Using the left hand for right femoral access, three fingers are placed over the femoral pulse proximal to the access site, and one finger distal to the access site.
- 2.
While applying firm pressure with the left hand, the right hand is used to remove the sheath from between the fingers of the left hand. The right hand can then be repositioned over the left hand to improve holding pressure and reduce fatigue.
- 3.
Occlusive pressure should be held for no more than 2–3 minutes, and then pressure slightly lessened for the remainder of the time used to hold pressure to allow for distal blood flow.
- 4.
The fingers should be straight to prevent the artery rolling out from under the fingers. This takes significantly more force than holding pressure with the fingertips. Pressure should be maintained firm enough to prevent any hematoma formation, which is detected as firmness around the compression site.
- 5.
After hemostasis is achieved, the distal pulses should be reexamined and compared with the baseline exam.
- 1.
The drawbacks to MC are that it can be time consuming, it can be physically challenging to hold adequate pressure to achieve hemostasis in very large patients, it may not work in fully anticoagulated or uncooperative patients, and it may be difficult to avoid vessel thrombosis in small arteries. It also requires postprocedure bed rest for 4–6 hours. This last reason has been a particularly important motivator in the development of vascular closure devices (VCDs), partly because the bed rest requirement can be quite difficult for certain patient populations (e.g., patients with orthopnea or musculoskeletal pain), sometimes even precluding them from a potential procedure if they cannot lie flat. Perhaps even more relevant is that in an ever-evolving cost-conscientious health system, decreasing the time to ambulation and expediting discharge times is increasingly desired.
Alternatives to Manual Compression
Hemostasis aids have been developed for multiple reasons and offer some advantages when compared to MC. Most of these aids were first designed to decrease the compression time needed to achieve hemostasis, and are now also often designed to reduce the requisite nonambulatory bed rest time and length of stay after achieving hemostasis. Another goal of these aids is minimizing complication rates associated with MC, particularly when procedure-specific or patient-specific factors increase the relative risk of bleeding, such as with larger arteriotomy sizes (sheath size >7–10F) or with overweight patients.
There are three broad categories of hemostasis aids: (1) compression devices, (2) topical agents, and (3) invasive devices. Invasive devices can be further broken down into those without and those with deployment of a foreign body inside or adjacent to the artery.
Compression Devices
Femoral compression devices include bedside C clamps and pneumatic compression straps (i.e., inflatable cuffs). The convenience of these devices is that the operator is freed to attend to other tasks while hemostasis is achieved. Because these devices do not alter the inherent underlying coagulation dynamics, the hemostasis success rate approaches near 100% in experienced hands with a complication rate similar to that of MC. Femoral compression devices are still used at some centers but have been either abandoned or replaced by invasive closure devices. However, the pneumatic compression devices for radial artery compression are widely considered the standard of care and appear to be as reliable as, or even more reliable than, MC.
Topical Agents
Topical agents are procoagulants applied to the puncture site at the time of MC. These agents are most suitable for superficial punctures, as might be encountered with radial artery access or dialysis fistula/graft access. They may shorten the time to hemostasis with MC or may even allow a sheath to be removed earlier than otherwise would be allowable in cases where systemic anticoagulation was administered. However, data on the efficacy or clinically relevant savings when using topical agents is debatable.
Invasive Devices
Most of these VCDs are designed for retrograde common femoral artery access. Of the devices that do not leave any foreign body after deployment, the more well-known is the Catalyst system (formerly Boomerang) (Cardiva Medical, Sunnyvale, CA). This device deploys an intraluminal “umbrella,” which is retracted against the arteriotomy to promote hemostasis and is then collapsed and completely withdrawn after a clot has formed outside the vessel. The theoretical advantage that this device has over the other invasive devices is a decreased risk of vessel stenosis or access site infection because no foreign material is deposited in the patient.
The much more commonly used VCDs actively close the arteriotomy, either by altering the pharmacologic or mechanical dynamics of hemostasis. Almost all these devices aim to instantly seal the arteriotomy by deposition of a procoagulant next to the artery or delivery of a suture or clip at the arteriotomy site. One of the more popular devices is the Angio-Seal vascular closure device (Terumo Medical, Somerset, NJ). This device seals the arteriotomy between an intraluminal resorbable footplate and a collagen plug deposited along the vessel surface. The device reports close to a 95% success rate, with decreased time to ambulation and no significant increase in the incidence of vascular complications. However, complications more specific to this device, particularly inflammation, infection, or the inadvertent deposition of the collagen plug in the artery, can be more severe in nature. Similar to this device is the Mynx catheter (Cordis, Milpitas, CA), which uses a removable balloon within the vessel lumen and a more inert sealant outside the artery. A review of some of the most common VCDs can be found in Table 8.1 .