The goal of sclerotherapy is to obliterate abnormal channels by damaging the endothelium, thereby resulting in subsequent inflammation and fibrosis. Venous malformations (VMs) are caused by abnormal development of the vein wall, with thinning and asymmetric disruption of the smooth muscle layer of the vein in association with endothelial cell abnormalities. This results in progressive, often asymmetric, dilation of the affected channels. Associated absence or insufficiency of valves in the conducting veins contributes to swelling. Affected channels become progressively enlarged, and the resulting stagnation of blood causes thrombosis, swelling, and pain. Most VMs undergo a continuous cycle of spontaneous thrombosis and thrombolysis. Calcification of thrombi results in formation of phleboliths. Symptoms and signs include blue or purple cutaneous lesions, swelling with dependency or effort, pain, deformity, and consumption coagulopathy. Pulmonary embolism can occur, especially when the conducting venous channels are malformed.
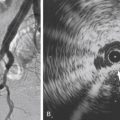
The angioarchitecture of VMs includes focal ( Fig. 17.1 ), multifocal ( Fig. 17.2 ; also see Fig. e17.1 ), and diffuse forms ( Fig. 17.3 ; also see Fig. e17.2 ). The degree to which the lesion communicates with adjacent conducting veins is the key factor in planning treatment. VMs with minimal communication can be considered “sequestered,” whereas those with free drainage are confluent or “nonsequestered” (see Fig. 17.3 and Fig. e17.2 ). Focal lesions may be intramuscular, cutaneous, or mucosal and usually consist of collections of abnormal interconnecting channels or spaces that are sequestered or drain through fairly small channels to normal adjacent conducting veins. This type of lesion is characterized by focal pain or a sensation of fullness with dependency, after exercise, or on arising in the morning (due to stasis). These lesions are easily and effectively treated by injection of sclerosant.
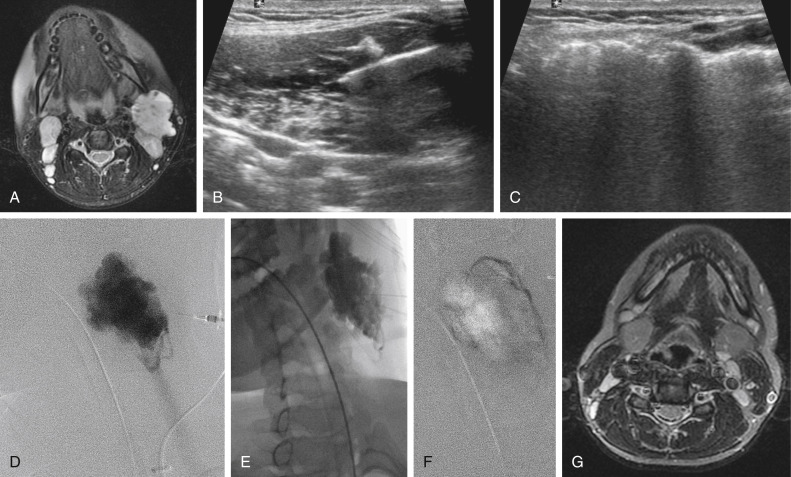
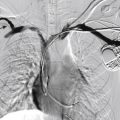
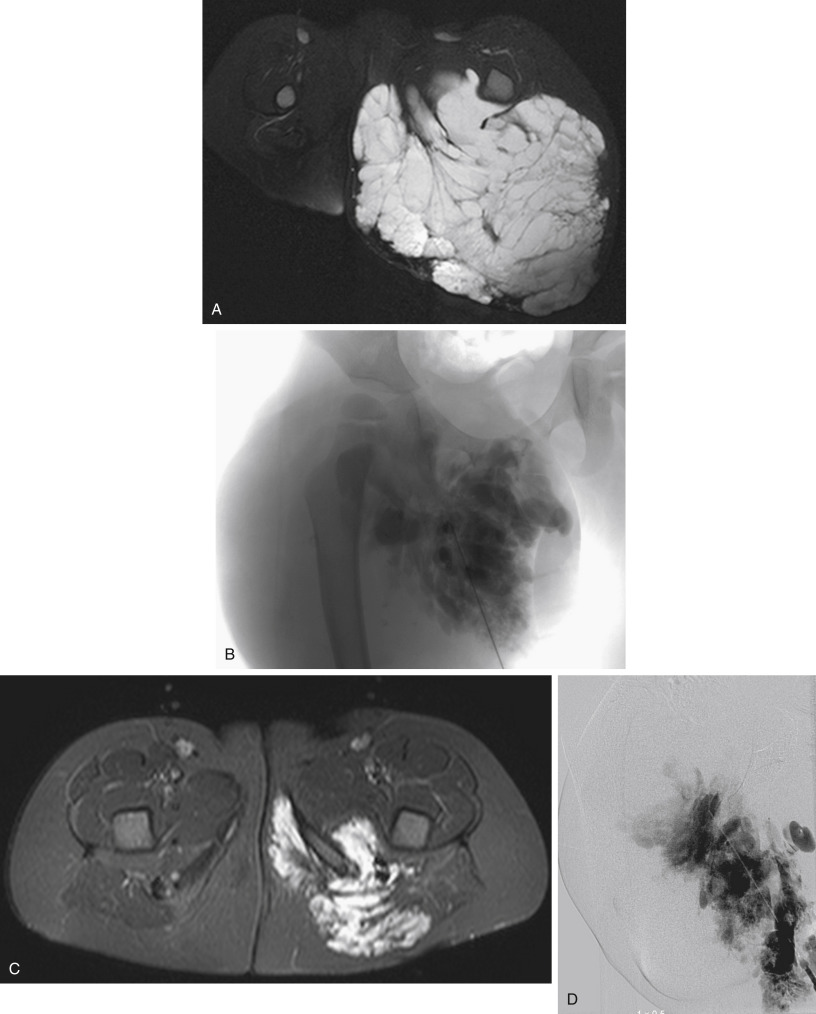
Diffuse VMs involve multiple tissue layers, usually including muscle, subcutaneous fat, skin, and sometimes bone. In diffuse lesions, the malformed veins are nonsequestered and communicate directly with the main conducting veins, which are frequently also abnormal. Diffuse VMs are difficult to treat effectively because injected sclerosant can directly enter the circulation and potentially cause deep venous thrombosis, pulmonary embolism, or systemic effects of ethanol. Recanalization is also more likely than after sclerotherapy for nonsequestered lesions. Some patients with diffuse VMs have focal eccentric varices that can exert a considerable mass effect on adjacent structures. These varices can be obliterated by endovascular treatment.
Multifocal lesions are most commonly seen in familial forms of VM, including blue rubber bleb nevus syndrome (see Fig. 17.2 and Fig. e17.2 ), mucocutaneous familial VMs, glomuvenous malformation, and Maffucci syndrome.
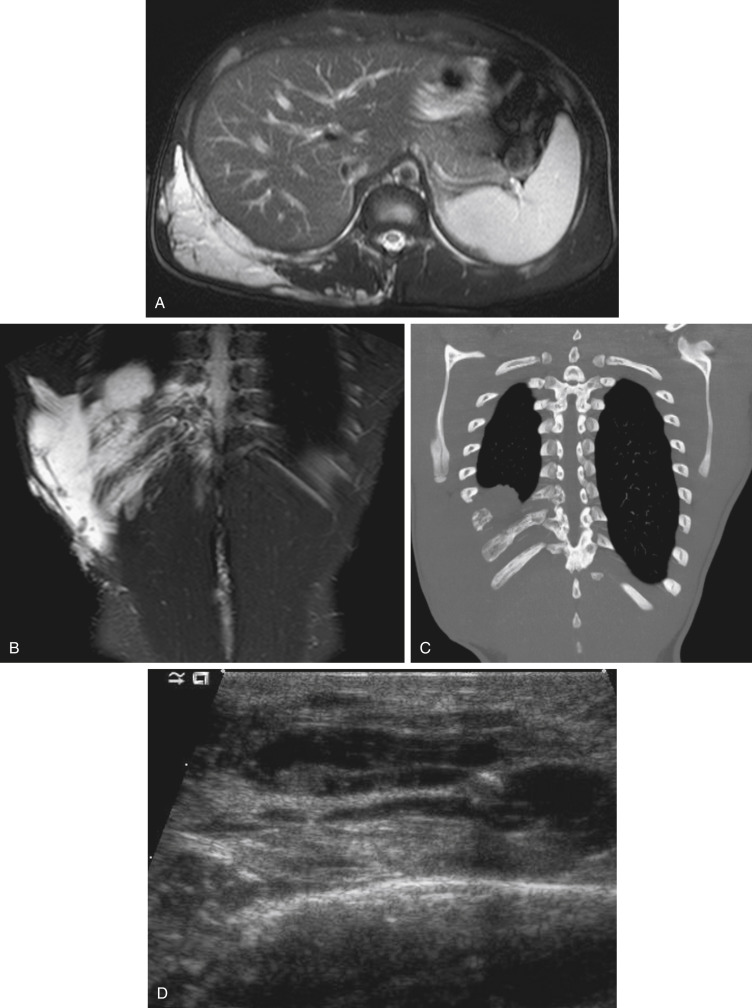
Lymphatic malformations (LMs) result from regional maldevelopment of lymphatic channels and include cystic and channel-type anomalies. Cystic LMs are generally classified into three groups: microcystic (cystic components <1–2 cm diameter), macrocystic, and combined forms. Macrocystic lesions are most common in the neck, axilla, and pelvis ( Fig. 17.4 ). LMs are frequently combined with cutaneous capillary malformations (capillary-lymphatic malformations [CLMs]) and anomalies of conducting venous channels (LM with venous dilation, lymphatic-venous malformations, or capillary-lymphatic-venous malformations [CLVMs]). Generally, LMs manifest either as focal mass lesions (macrocystic) or as diffuse tissue swelling or overgrowth. Unlike VMs, they do not expand with the Valsalva maneuver but rather expand or swell intermittently, especially in association with systemic viral illness. Swelling can occur acutely as a result of infection or bleeding into the lesion. Bleeding is presumably due to either adjacent abnormal venous channels or rupture of small arteries, frequently seen in the septa. Sepsis is most frequently a problem with lesions close to the alimentary tract (e.g., face, pelvis). Cutaneous extensions of LMs are manifested as vesicles that may leak clear, bloody, or chylous fluid. Diffuse and multifocal LMs (generalized lymphatic anomaly) are composed of incompetent lymphatic channels, often associated with chylous reflux and leaks. Life-threatening chylothorax or chylous ascites may develop in patients with generalized lymphatic anomaly. Gorham syndrome (vanishing bone disease) is a type of CLM associated with increased osteoclastic activity and progressive osteolysis of affected bone, most often in the shoulder and pelvis.
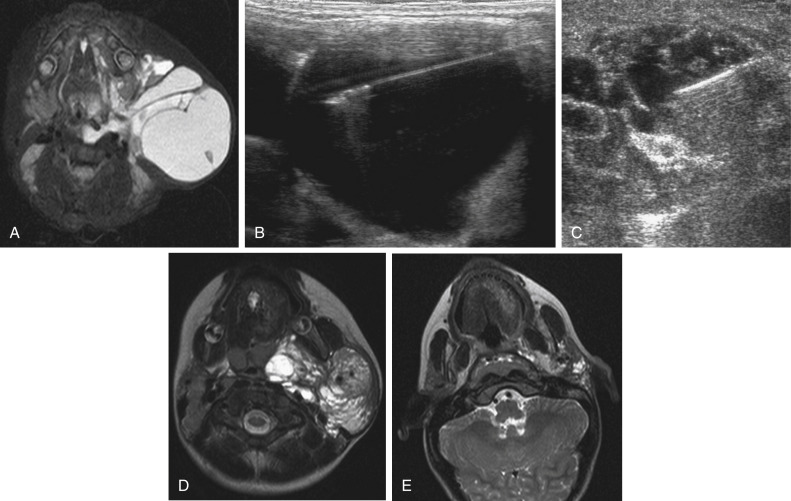
Magnetic resonance imaging (MRI) is the best technique to confirm the diagnosis and extent of low-flow vascular malformations. VMs and LMs are hyperintense on fluid-sensitive sequences and do not have fast-flow components on gradient-recalled echo sequences. VMs enhance inhomogeneously, whereas LMs typically show minimal or rim enhancement. Arteriography is not routinely necessary.
Indications
Endovascular or percutaneous treatment is warranted in patients with significant symptomatology not well controlled by conservative treatment. For VMs, standard nonoperative treatment includes use of graded elastic compression garments (for limbs) and aspirin or anticoagulation. Indications for invasive treatment include pain secondary to swelling, a mass effect resulting in functional impairment (e.g., interference with ambulation, breathing, swallowing, speech), and disfigurement. For macrocystic LMs, sclerotherapy is now the preferred treatment over resection. Indications include pain, recurrent infection or bleeding, and a significant mass effect in the presence of macrocysts on imaging.
Contraindications
Pulmonary hypertension and atrial septal defects are contraindications to sclerotherapy for VMs unless the malformation is completely sequestered from the venous circulation. Relative contraindications include diffuse involvement of a closed space, such as the orbit or a muscle compartment (because of the risk of compartment syndrome), confluent or nonsequestered VMs, and severe consumption coagulopathy. The latter can often be corrected or improved with use of anticoagulants for 2 weeks before treatment. In patients with vascular malformations involving or adjacent to the airway, precautions for airway protection must be taken. Patients who have had cardiovascular complications from ethanol should not receive this agent again. Those with lesion-related neuropathy may be made worse by sclerotherapy.
Equipment
Low-flow vascular malformations are best treated in an angiography suite with road-mapping capability and anesthesia support. Ultrasound equipment with high-frequency probes and sterile probe covers and gel is useful. An automated tourniquet system with sterile cuffs is important for control of flow in some VMs of the limbs. Sclerosants include 95% to 98% ethanol, 3% sodium tetradecyl sulfate (STS), morrhuate sodium, ethanolamine, and bleomycin for VMs, and doxycycline, OK-432, bleomycin, or ethanol for LMs. Ethiodol or Lipiodol can be used to opacify sclerosants with minimal dilution. An agent that combines ethanol and ethyl cellulose for greater viscosity has been developed but is not yet clinically available. Useful needles include angiocatheters, 21-gauge single-wall needles, and 25- and 27-gauge butterfly needles. Plastic connecting tubes and three-way stopcocks are useful for hand-injected imaging. If oily contrast medium is used, plastic stopcocks must be checked for compatibility (see Fig. e17.2 ).
Technique
Vascular Malformations
Anatomy and Approach
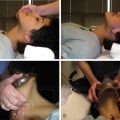
Sclerotherapy is performed by direct percutaneous cannulation of vascular channels, usually with a simple needle. Previously obtained MRIs are used to determine the location and extent of the lesion and are available in the procedure room to help plan the percutaneous access. Sonographic guidance of needle placement is useful (see Fig. 17.1B ). MRI guidance is feasible and may be advantageous for deep lesions not easily seen with sonography. Small localized lesions can be injected with detergent sclerosants under regional anesthesia. Patients with extensive VMs and those undergoing ethanol sclerotherapy are anesthetized and pretreated with corticosteroids, and have a Foley catheter placed. Patients undergoing sclerotherapy for VMs must be kept well hydrated to compensate for the hemolysis caused by the sclerosant.
Technical Aspects
Affected limbs are widely prepared to permit observation of the skin and placement of a peripheral intravenous cannula and tourniquet or other occlusion device.
Compression of proximal veins is useful to distend the malformation before cannulation. The abnormal channels or spaces are cannulated with a needle after localization by either palpation or sonography. After obtaining blood return, contrast medium is injected into the lesion, followed by sclerosant. The process is repeated until the selected components of the malformation are all injected.
Contrast medium is injected, usually with imaging by digital subtraction radiography, to exclude arterial cannulation or extravasation, assess the nature of the lesion and its drainage, determine how much of the lesion is accessed, and calculate how much liquid is necessary to displace the blood into the draining vein. If possible, blood is displaced from the malformation. The appropriate sclerosant drug is then injected. The initial volume injected is less than the amount of contrast medium required to opacify the draining vein. Ethanol should not be used in lesions adjacent to major nerves or cutaneous lesions or in confluent or nonsequestered venous channels without some form of outflow control. The total volume of ethanol injected in one session should be less than 0.5 mL/kg or 40 mL. The volume of 3% STS should be kept below 0.5 mL/kg or 20 mL per session. STS can be diluted with normal saline (e.g., 0.5%–1% STS) for injection into cutaneous lesions.
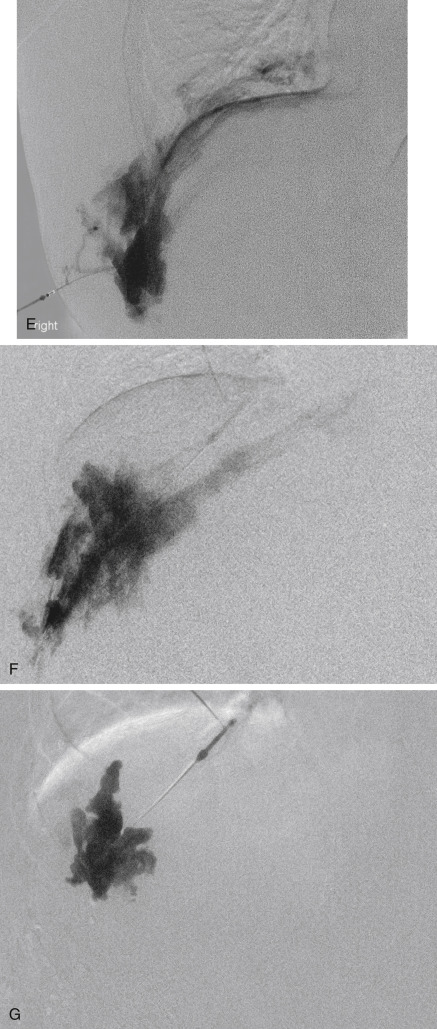
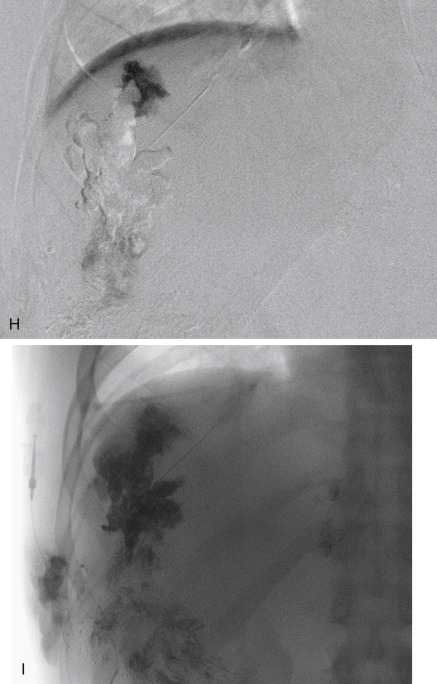
Sclerosing agents may be opacified for fluoroscopic guidance. Detergent sclerosants can be mixed with either oily or water-soluble liquid contrast. Oily contrast medium (Ethiodol or Lipiodol) added in a 1:10 to 3:10 ratio (contrast to sclerosant) results in minimal dilution and, when combined with detergent sclerosant and air mixed through a three-way stopcock, produces relatively stable foam. The injection of opacified sclerosant should be observed by fluoroscopic subtraction (road map); injection must be stopped if extravasation or arterial penetration is seen. Once the sclerosant reaches a small outflow vein, injection should be stopped for a few minutes to allow occlusion of the outflow. Additional opacified sclerosant is injected until the lesion is completely filled as shown by fluoroscopy. With nonopacified sclerosant, roadmap imaging shows washout of previously injected contrast or negative opacification (see Fig. 17.1F ). Control of minor documented venous drainage to minimize egress of sclerosant can be accomplished in the head and neck by manual compression, and in the limbs by manual compression or tourniquets. A sterile automated orthopedic tourniquet inflated to a pressure less than mean arterial pressure can be used in the limbs. In lesions with significant venous drainage (confluent VMs), permanent outflow occlusion—accomplished by placing coils or tissue adhesive at sites of communication between the malformation and adjacent conducting veins—is useful in retaining the sclerosant in the VM and minimizing the risk for pulmonary embolism or cardiovascular reaction to the sclerosant (see Fig. e17.2 ). When sclerosing VMs of the extremities, a peripheral intravenous line should be placed in the affected limb to document venous patency before and after treatment and to infuse heparinized saline during sclerotherapy.
Sclerotherapy for superficial vascular malformations is performed with unopacified (sometimes diluted) sclerosant, with observation of skin color rather than the fluoroscopic image. The injection must be terminated when ischemic changes such as pallor or duskiness become evident. Applying cold sterile saline onto the surface of the skin to induce local vasoconstriction seems to minimize damage.
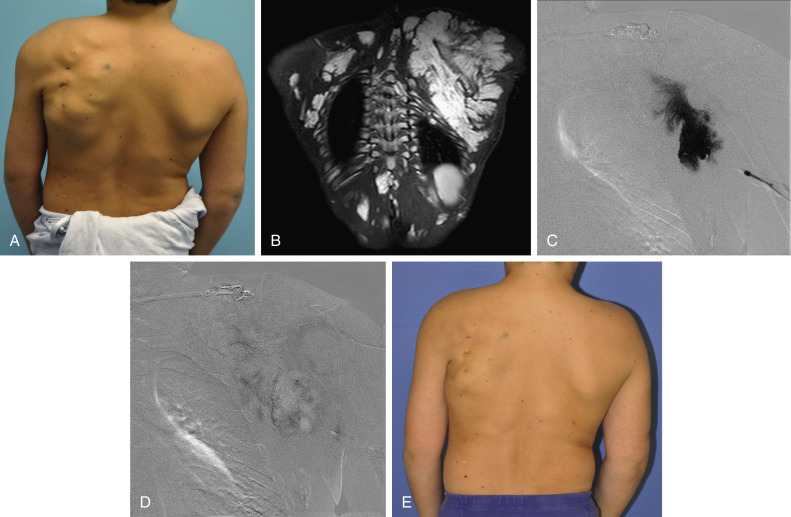
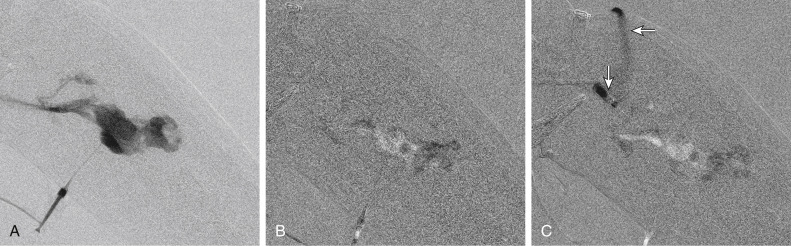
A double-needle technique in which a venous channel is cannulated with two needles, one for injection and one to release blood and excess sclerosant, has been proposed for added safety (see Fig. 17.3 ).
Adjunctive Techniques
Use of Platinum/Fiber Coils
In the presence of rapid venous drainage or massive venous spaces, direct injection of sclerosant into the VM is both ineffective and risky. In this type of lesion, placing coils into the malformation or at the junction of the malformation and the draining veins before injection of sclerosant creates a permanent sclerosis and minimizes the risk for pulmonary thromboembolism. Microcoils can be placed into the venous pouch directly through the access needle, with or without control by a balloon occlusion catheter introduced via the femoral or internal jugular veins, or a tourniquet. Sclerosant can be injected around the coils. In extensive malformations of the conducting veins, such as the marginal varices in patients with Klippel-Trenaunay syndrome CLVM, a catheter or microcatheter can be placed percutaneously into the vein and advanced to the confluence between the anomalous and deep veins or perforators. After occluding the anastomoses with coils, the sclerosant can be injected along the length of the anomalous vein as the catheter is withdrawn.
Use of Acrylic Polymer
Acrylic polymers such as N -butyl-2-cyanoacrylate (NBCA) are not ideal sclerosing agents for VMs. In addition to their high cost, they are absorbed slowly, and a firm painful mass is left in the soft tissues for many months. They are useful when rapid occlusion and minimal swelling are desired, such as for outflow occlusion in conjunction with injection of sclerosant or for hemostasis before resection (see Fig. e17.2 ). NBCA opacified with oily contrast medium in a ratio of 1:4 to 1:6 appears to be effective in controlling bleeding and improving localization of VMs during resection. Risks include unintended occlusion of major veins and pulmonary embolization. Intraoperative injection of tissue adhesive (NBCA) immediately before surgical excision of VMs of the orbit has been reported. In general, orbital VMs should not be treated by sclerotherapy because of the potential for development of orbital compartment syndrome and subsequent loss of vision. An exception to this rule is use of bleomycin, which causes minimal swelling.
Intralesional Laser Photocoagulation
Diode or neodymium:yttrium aluminum garnet bare laser fibers can be used through catheters or large cannulas to ablate the endothelium of VMs. This procedure is especially useful for treating the long anomalous channels in patients with CLVMs, but it can also be applied to common VMs (see Fig. e17.2 ). It appears to be most effective when combined with injection of sclerosant. Compared with sclerotherapy alone, endovenous laser therapy /sclerotherapy results in less postprocedural swelling and faster recovery.
Bleomycin
Bleomycin (1 unit/mL, maximum 15 units per procedure) is a useful sclerosant in selected lesions. It results in progressive fibrosis of the VM, without acute thrombosis and with minimal swelling, making it the best agent for VMs in the orbit or airway. As a sole sclerosant, it should be reinjected every 3 weeks for optimal results. Bleomycin can also be used with a small amount of ethanol or foam after outflow occlusion (see Fig. 17.1 ). This technique causes lesional thrombosis, so procedures are staged 6 to 8 weeks apart. Side effects of bleomycin include alopecia, pigmentation, nausea, hair loss, pulmonary fibrosis, acute pulmonary reaction (acute respiratory distress syndrome), and anaphylaxis.
Lymphatic Malformations
Sclerosants used for LMs include ethanol, doxycycline, bleomycin, Ethibloc, and OK-432. The latter two agents are not approved for use in the United States. OK-432, a solution of killed group A streptococci in a suspension containing penicillin, is undergoing clinical evaluation in a multicenter head and neck trial under the supervision of the University of Iowa. It has been used extensively in Japan, Canada, and Europe. OK-432 causes an inflammation, probably due to an immune response to the agent, and subsequent shrinkage of the cysts (see Fig. 17.4 ).
Bleomycin is effective in treating LMs, although because of potential systemic toxicity it is best to reserve its use for LMs that have failed other sclerosants or for microcystic LMs in the orbit or airway. Ethanol can be used for localized macrocystic LMs, but has the highest risk of nerve injury and skin necrosis. Doxycycline is effective in extensive LMs, because large volumes given at one session are well tolerated (see Fig. e17.3 ). It is generally administered as a solution of 10 mg/mL in volumes up to 100 mL. The injection is extremely painful during and for 2 hours after injection. Reconstitution of the drug with a mixture of water and contrast medium results in radiopacity. STS is used for sclerosing microcysts and cutaneous vesicles. Acetic acid has also been reported to be an effective sclerosant for LMs but is not widely used.
Individual macrocysts are cannulated with a needle under sonographic guidance, and fluid is aspirated as completely as possible. Alternatively, pigtail catheters can be placed and used to inject and drain the cysts sequentially over a period of several days. The cysts can be opacified with a small amount of contrast medium, which is then aspirated, or needle position can be confirmed by ultrasonography alone. Injection of sclerosant can be monitored with ultrasonography or fluoroscopic guidance. For superficial lesions consisting of smaller cysts, sonographic guidance is more practical and effective. The needle, connected to a syringe of sclerosant, is positioned in a cyst or space, sclerosant is injected, and the needle is moved to another cyst and the process repeated. If additional treatment is necessary, injection is usually repeated every 6 weeks. A multiagent technique has also been described in which a pigtail catheter is placed, and a cyst is injected first with STS, then with ethanol. Although effective in sclerosing individual cysts, this technique requires pigtail catheter drainage for several days.
Controversies
Choice of Sclerosant
Ethanol has been assumed to be more effective than detergents in sclerosing VMs. However, recanalization and recurrence occur with all agents. Ethanol is more likely to result in severe complications, including neuropathy, tissue necrosis, cardiovascular collapse, and death. Since foamed detergent was shown to be more effective than liquid, many centers have decreased their use of ethanol for VMs.
Bleomycin, alone or in combination with other sclerosants, results in effective sclerosis and less swelling than other agents. Because of the risk of pulmonary fibrosis, some believe it is not an appropriate drug for benign vascular malformations.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree