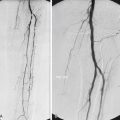
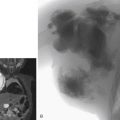
Clinical Relevance
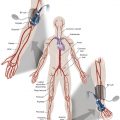
Abdominal aortic aneurysms (AAAs) occur in approximately 5% to 10% of the male population older than 65 years, and the incidence increases with advancing age. The risk of rupture is very low for aneurysms less than 5 cm in diameter, but increases substantially with aneurysms larger than 6 cm in diameter. Mortality rates for traditional open repair of AAAs vary widely, depending on the operator’s experience. In the United Kingdom, the overall in-hospital mortality rates for open and endovascular elective treatment were, respectively, 3.1% and 0.6% in a 2017 report. It is higher in emergency cases.
Endovascular aneurysm repair (EVAR) was initially introduced with the aim of achieving lower mortality and morbidity rates in elderly and unfit patients deemed to be at greater risk for complications from conventional surgery. Since its introduction by Parodi almost two decades ago, EVAR has become widely used worldwide and is now the main method used to treat many patients with AAAs.
Indications
Selection of patients suitable for standard EVAR is usually made in a multidisciplinary context, weighing patients’ risk factors such as age > 70 years, coronary artery disease, respiratory disease, renal function, obesity, and the anatomic scenario.
Coronary artery disease in particular is considered the leading cause of mortality after open surgery because it is associated with high (>5%) risk of perioperative cardiovascular complications (myocardial infarction, stroke, and cardiac death).
The risk of contrast-induced nephropathy should also be taken into account, particularly in patients with an estimated glomerular filtration rate < 30 mL/min, diabetes mellitus, old age, reduced left ventricle systolic function, advanced heart failure, acute myocardial infarction, and shock.
Indications for treatment of AAAs with EVAR are the same as for conventional surgical repair.
- •
Aneurysm diameter. Treatment of AAAs is based on the aneurysm reaching a size threshold above which leaving it untreated is more hazardous than treatment. The treatment threshold is based on the perceived annual rupture rate of aneurysms of different diameters. Although rupture rates of small aneurysms are very low, the rate increases to 10% per year once aneurysms reach 6 cm in diameter. The UK Small Aneurysm Trial (UKSAT) and the US Aneurysm Detection and Management (ADAM) study were conducted to discern optimal management of AAAs between 4.0 and 5.5 cm. Both studies concluded that surgery should be performed at a threshold diameter of 5.5 cm, and that surgery for smaller aneurysms provided no survival advantage. It remains controversial whether the 5.5-cm threshold should apply to women, and many interventionalists treat AAAs in women when they reach 5 cm, even less in some centers.
- •
Symptomatic aneurysms. Painful or tender AAAs.
- •
Rapidly growing aneurysms. AAAs with an increase in diameter ≥ 5 mm within 6 months.
- •
Anatomic Inclusion Criteria for Endovascular Aneurysm Repair
Inclusion criteria for EVAR are based mainly on the anatomic features of the proximal aortic neck (distance between the lower renal artery and the aneurysm) to achieve a good sealing and of the iliofemoral axis for a safety introduction and removal of the stent-graft device.
Neck Inclusion Criteria
Neck Diameter
In general, the maximum diameter of the proximal aortic neck, for conventional EVAR, is 33 mm. Distal aortic neck should be greater than 18 mm to accommodate the two stent-graft limbs.
Neck Length
The standard required length of the proximal neck (distance between the lowest renal artery and the aneurysm sac) is 15 mm. A shorter neck can be managed using devices with suprarenal attachment, fenestrated or branched stent-graft, and chimney technique. However, the potential for a proximal leak increases with decreasing neck length.
Neck Angulation
Increasing angulation reduces the success of fixation and attainment of an adequate seal. The accepted threshold of angulation is 60 degrees. However, EVAR is often successful in necks angulated by more than 60 degrees if neck length is longer than 15 mm. Also, a severe neck angulation increases the potential for a proximal leak.
Neck Shape
Proximal aortic necks that increase in diameter inferiorly (conical shape) or superiorly (reverse conical shape) decrease the potential for adequate fixation and seal. Suprarenal fixation is highly recommended to achieve a good sealing.
Mural Thrombus and Calcification
In general, increasing amounts of mural thrombus or calcification increases the potential for a proximal leak and for stent-graft migration. For this reason they are considered by many as a contraindication to EVAR. However, suprarenal fixation can reduce the risk of stent-graft failure.
Iliac Artery Inclusion Criteria
Diameter and Length
The maximum iliac diameter that can act as a distal landing zone for EVAR depends on the maximum device diameter available. In practice, this is currently 25 mm (Endurant device, maximum limb diameter = 28 mm [Medtronic Inc., Santa Rosa, CA]).
In those cases where the diameter of the common iliac artery (CIA) is too big for the available devices, the aortic aneurysm involves the CIA, or the CIA length is less than 3 cm, there are several options to achieve a correct sealing. Extending the endograft into the external iliac artery, which generally presents a smaller caliber (8–10 mm), is the most popular and easiest option. The internal iliac artery (IIA) must be occluded, using coils or vascular plug, to avoid retrograde endoleak. However, this technique can be associated, especially in older patients and in cases of occlusion of the contralateral IIA, with ischemic complications such as buttock claudication. Another option is the use of iliac branched devices, which allow continuous perfusion of the IIA. However, this technique is technically challenging, with an increased operating time (up to 390 minutes) and relatively low technical success rates. In addition, some anatomic limitations, due to graft sizing, must be considered for this technique. For example, for the Excluder iliac branch endoprosthesis (W.L. Gore & Associates, Flagstaff, AZ), maximum sizing is: IIA range, 6.5–13.5 mm; external iliac artery diameter range, 6.5–25 mm; maximum CIA branch component length, 10 cm.
The femoroiliac axis must also be of adequate caliber (>7 mm) to accept passage of the endograft (minimum aortic main body device, 18F, 6 mm).
Tortuosity and Calcification
It may not be possible to pass the delivery catheter up excessively tortuous iliac arteries. However, many noncalcified tortuous iliac arteries can be straightened out by very stiff guidewires, but this is seldom possible if the iliac arteries are heavily calcified.
In cases of severe calcification of the iliac axis, the “crack and pave” technique can be used. After deployment of a covered stent-graft, of a caliber sufficient to allow safe introduction of the aortic device, the covered stent-graft is postdilated with a large balloon. Potential vessel rupture and fissuration are protected by the presence of the stent-graft.
Contraindications
There are very few absolute contraindications to EVAR, and all are related to the vessel’s morphology.
Imaging
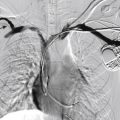
All patients require comprehensive imaging of the aorta and iliofemoral arteries to assess their suitability for EVAR. In particular, characteristics of the proximal and distal necks, the length of the aorta and iliac arteries, the vessels’ diameters and the vessels’ morphology (e.g., calcifications, mural thrombus) must be evaluated.
The mainstay of imaging is computed tomography angiography (CTA), which provides optimal information on the lumen and wall of the aortoiliofemoral segment. It also allows easy multiplanar reconstruction and vessel tracking.
Most recent application of CTA in EVAR planning is represented by the use of 4D-reconstruction and 3D printing. The advantages of a 3D physical model would be a better representation of the anatomy, especially in cases of severe tortuosity. Moreover, this may be useful in the planning of stent-assisted EVAR (chimney, periscopes), fenestrated EVAR (FEVAR), or branched EVAR. In fact, recent studies reported significant changes in the procedure planning especially in the hostile neck and tortuous iliac arteries: in a retrospective analysis of 144 cases, following 3D model review, the trend routed slightly toward more open procedures (EVAR dropped from 73.6% to 67.4%) and off-label techniques reduced from 19.4% to 15.2%.
However, in patients with impaired renal function, magnetic resonance (MR) imaging has been considered a valid alternative for preprocedural planning. In fact, unenhanced MR angiography (MRA) avoids ionizing radiation and is especially useful for patients with poor renal function who are not undergoing dialysis. Moreover, recent studies showed no difference in the stent size and configuration chosen when unenhanced MRA was compared with CTA. Limitations of MRA lie in the detection of small accessory renal arteries that may be missed and in the evaluation of the degree of calcifications. Therefore, when performing MRA, a plain CT is generally advised to visualize calcified disease, especially in the landing zones and along the iliofemoral axes.
Regardless of the imaging modality, the following parameters should be assessed: diameter and length of the proximal neck, maximum diameter of the aneurysm sac, diameters of the iliofemoral arteries, and length of the common iliac arteries.
Device Selection
Endograft must be selected on the basis of the anatomic characteristics. However, in many institutions, selection of a particular device is often decided by the personal preference and experience of the operator.
Due to the recent advancements in technology, a growing number of AAAs can be treated with endovascular techniques, even in more complex scenarios. In particular, it has been estimated that up to 70% of patients may be suitable for conventional EVAR using new generation endografts.
Kontopodis et al. investigated anatomic suitability to standard EVAR comparing the different new stent-grafts commercially available. EVAR eligibility resulted to be higher for the Ovation system (72%) compared with the rest of the devices: Incraft 63%, Nellix 60%, Endurant II 59%, Excluder 55%, Zenith Flex 36%, and Aorfix 35% ( P < .001). Nonsuitable proximal neck anatomy followed by access vessel inadequacy were found to be the primary reasons for ineligibility.
However, long-term durability is still being debated, especially in cases of adverse anatomy, making the preoperative evaluation and choice of the appropriate stent-graft paramount for late success of EVAR. In fact, problems such as stent-graft migration/disconnection, late type I, II, and III endoleaks, early/late limb occlusion, and post-EVAR aortic neck enlargement (due to chronic outward force) still remain a concern.
According to the instructions for use (IFU) of the commercially available standard stent-grafts, main anatomic features and indications may vary according to graft model.
Endografts are divided in aortouniiliac, modular bifurcated (with two or more components) with or without suprarenal attachment, branched EVAR, and FEVAR.
Tables 20.1 and 20.2 describe all the stent-grafts commercially available.
| Stent-Graft | Materials | Flexibility and Conformability | Deployment | Access (Main Body) | Durability | Special Feature |
|---|---|---|---|---|---|---|
| Endurant II (Medtronic) | PTFE and Nitinol stents, suprarenal fixation | Neck < 10 mm and < 60 degrees Or >15 mm and 75 degree angle | Controlled-release system; top cap to be recaptured and retrieved | Sheathless 18–20F OD | ENGAGE registry: 97.8% freedom from aneurysm-related mortality and 89.4% stable or decrease in AAA sac diameter. | CE approval for use in chimney EVAR and endoanchors. |
| Zenith and Zenith Alpha (Cook Medical) | Woven polyester – Nitinol (Alpha) Stainless steel (earlier Zenith stent-grafts) Suprarenal fixation Integrated sheath | Neck length/angle Suprarenal fixation | Easier deployment in three steps. The top cap was eliminated from the delivery system, which allowed a further reduction in overall profile. | 18F OD | Sobocinski et al.: Zenith Alpha vs. Zenith: The first group did not demonstrate a higher incidence of limb occlusion (1.3% vs. 3.6%) or endoleak (5% vs. 8%) during follow-up. There was no incidence of sac expansion. | Custom-made devices available, also for FEVAR. |
| Incraft (Cordis, Hialeah, FL) | Low-porosity polyester and segmented Nitinol stents | 14 OD Neck no shorter than 10 mm and more than 60 degree angle | Controlled-release system; no top cap to be recaptured | 14F OD Can be sheathless | INNOVATION prospective multicenter trial: no type I or III endoleaks at the 2-y mark. All-cause mortality at 2 years was 11.5%, and no death was device or procedure related. | Suitable for tortuous iliac arteries. Attractive for PEVAR. |
| AFX (Endologix, Irvine, CA) | STRATA ePTFE externally mounted at the proximal and distal ends of a cobalt chromium stent | The “ActiveSeal” results in the graft material conforming to the neck and aortic bifurcation. Neck length >15 mm and angle less than 60 degrees still apply. | Four-component device, including a proximal cuff with suprarenal fixation and bilateral iliac limb extensions | 19F OD | 2.3% type Ia endoleaks, 0–2.3% type III endoleak, and about 5% type II endoleaks, at 10–20 mo follow-up. | Preserves aortic bifurcation. Single lumen at aortic bifurcation (can use for small distal aorta). These features prevent distal migration. |
| Ovation stent-graft (Endologix ) | PTFE and Nitinol stents Larger suprarenal stent fixation system | Suitable for hostile AAA neck and tortuous anatomy Inflatable channels and sealing O-rings in the aortic body that are filled with a polymer. Suitable for necks as short as 10 mm with angulation up to and including 60 degrees and >10 mm long neck if the neck angle is less than 45 degrees. | Trimodular design | 14–15F | Freedom from type Ia endoleak (98.2% IFU vs. 96.8% OU, P = 0.6; HR 0.55, 95% CI 0.02–9.71) or freedom from any device-related reintervention (92.8% IFU vs. 96.4% OU, P = 0.4; HR 2.42, 95% CI 0.–12.99), at 24 months follow-up. | O-rings sealing system does not create chronic outward forces, resulting in no aortic neck expansion. |
| Altura (Lombard Medical, UK) | Ultra-low-porosity woven polyester graft with endoskeleton Braided Nitinol stents | Neck length >15 mm and angle less than 60 degrees. Low profile allows for easy positioning and deployment in tortuous iliac arteries. | Possible repositioning during deployment and accurate graft placement at each renal artery No contralateral limb cannulation | 14F | Freedom from type I/III endoleaks in 95% of patients at 1 y. | Shorter fluoroscopy and procedure time. Potentially suitable for outpatient EVAR. |
| Treovance (Bolton) | Nitinol stents sutured to woven polyester | Neck < 10 mm and < 60 degrees Or >15 mm and 75 degree angle | Navitel delivery system: Suprarenal and infrarenal fixation hooks Proximal clasp allows repositioning | 18–19F | During a mean 12-mo follow-up (range, 6–24 mo), clinical success was 97%. No type I or III endoleak, death, AAA rupture, open conversion, or device-related serious adverse events were documented. Few ( 8.7%) patients required reintervention. | Custom-made device available. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree